
����ͼ��ʾװ�ý���ʵ��(�г�װ������ȥ)����ش��������⣺
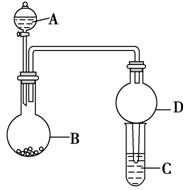
(1)��A��ΪŨ���ᣬB��Ϊ�����ʣ�C��Ϊ����������Һ����Ũ�������B�У���B�н�����________������Ԫ�������ڱ��е�λ��Ϊ____________________����̬��ԭ�Ӻ�������Ų�ʽΪ________________________________________________________________________��
(2)��A��ΪŨ���ᣬB��Ϊͭ���ʣ�C��Ϊ����������Һ����Ũ�������B�У���B�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
(3)��A��ΪŨ��ˮ��B��Ϊij���壬C��Ϊ������Һ��
�ٽ�Ũ��ˮ��ε���B�У��ɲ���������������B�й��������________(�����)��
a��CaO���������������� b��NaOH
c��NaCl d��P2O5
��C��ͨ���������ʱ����Ӧ�����ӷ���ʽΪ________________________________��
(4)��A��Ϊϡ���ᣬB��Ϊ̼��ƣ�C��ΪNaAlO2��Һ����ϡ�������B�к�B�з�Ӧ�����ӷ���ʽΪ_____________________________________________________________��
������HCl�Ļӷ�����������������������C�з�����Ӧ�����ӷ���ʽΪ________________________________________________________________________��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£���һ������ˮ���հ������Ƶ�Ũ��Ϊ12.0mol/L���ܶ�Ϊ0.915g/cm3�İ�ˮ���Լ���1�����ˮ���ն�������İ������Ƶ�������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����ʵ������ȡCl2ʱ���ܽ������²�����
�����Ӻ�װ�ü�������ԡ��ڻ������ȡ��ۼ���MnO2��ĩ�����ɷ�Һ©������ƿ���Ũ���ᡡ�ݶ����Cl2��NaOH���ա����������ſ������ռ�Cl2������ȷ����˳����
A���٢ڢۢܢݢ� B���ۢܢڢ٢ޢ�
C���٢ۢܢڢޢ� D���ۢ٢ܢڢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڻ�������������Դ�������У�����ȷ����
A�������������ǹ⻯ѧ��������Ҫ��Ⱦ�������̼������ЧӦ����Ҫ��Ⱦ��������ǵĺ����ǿ��������������Ҫ��Ŀ
B���ϸ���ͨ�������һ�ֲ������εķ���Ⱦ�ֶΣ���Ϊʵ��������к�����û�еõ�ת��������
C��������������Դ���������ù�ҵ�����ķ��ȣ��ǻ�����ԴΣ������Ҫ;��
D���ö��������������ơ��յ���Ȼ�ѧҩƷ�����������ʳ�ζ����ɫ�ޡ��������Ӽ����꣬������Σ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ȵ�ľ̿��Ũ���Ṳ�Ȳ���������ȷ�Ϊ�ٺ͢����ݣ��ڢٷ���ͨ����������ˮ���ٵ�������ʯ��ˮ���ڢڷ�ֱ��ͨ������ʯ��ˮ����ʯ��ˮ�ı仯�����Ϊ
A���ٲ�����ǣ��ڱ����ɫ
B���ٱ����ɫ���ڲ������
C���ٱ����ɫ���ڱ����ɫ
D���ٲ�����ǣ��ڲ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�����ȡ����Ӧ����(����)
��CH3CH===CH2��Br2 CH3CHBrCH2Br
CH3CHBrCH2Br
��2CH3CH2OH��O2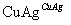 2CH3CHO��2H2O
2CH3CHO��2H2O
��CH3COOH��CH3CH2OH
CH3COOCH2CH3��H2O
��C6H6��HNO3 C6H5NO2��H2O
C6H5NO2��H2O
A���٢� B���ۢ�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ4���廷��ϩ��������4����ͬ��Ӧ�����У�����ֻ����һ�ֹ����ŵķ�Ӧ��(����)

A���٢ڡ���������������B���ڢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£���Һ������Զ�TiO2���Ⱦ��R���ⷴӦ��Ӱ�� ����ͼ��ʾ�������ж���ȷ����( )
A����0-50 min֮�䣬 pH = 2��PH = 7ʱR�Ľ���ٷ��ʲ����
B���� 20-25 min֮�䣬 pH = 10 ʱR��ƽ����������Ϊ 0.04 mol•L-1•min-1
C����Һ����Խǿ�� R �Ľ�������ԽС
 D��R����ʼŨ�Ⱥ���Һ��PH��Ӱ��R�Ľ�������
D��R����ʼŨ�Ⱥ���Һ��PH��Ӱ��R�Ľ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com