
| 1 |
| 2 |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����֪c��ʯī��s��=c�����ʯ��s����H��0������ʯ��ʯī�ȶ� |
| B����֪H2��g��+F2��g���T2HF��g����H=-270kJ/mol����2L����������ֽ��1L������1L��������270kJ���� |
| C��HCl��NaOH��Ӧ���к���Ϊ-57.3kJ/mol����H2SO4��Ba��oH��2��Ӧ���к��ȡ�H=2����-57.3��kJ/mol |
| D����֪I2��g��+H2��g���T2HI��g����H1��I2��s��+H2��g���T2HI��g����H2�����H1����H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
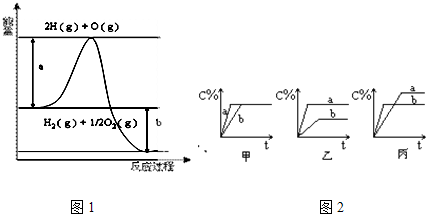
A��H2��g��+
| ||
| B��2H2��g��+O2��g���T2H2O��g����H=2��b-a��kJ?mol-1 | ||
C��H2��g��+
| ||
| D��2H2��g��+O2��g���T2H2O��l����H=2��a-b-c��kJ?mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
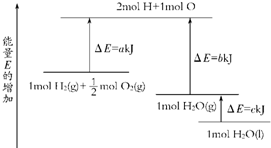
| 1 |
| 2 |
| 1 |
| 2 |
| A�����Ƕ������ȷ�Ӧ | B��a��b��c��Ϊ��ֵ |
| C��a=b? | D��2b=c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ƿ��ȷ�Ӧ�����Է��ģ��������ȷ�Ӧ���Ƿ��Է��� |
| B���Է���Ӧ��ǡ�������²���ʵ�� |
| C���Է���Ӧ���κ������¶���ʵ�� |
| D���Է���Ӧһ�����������Է���Ӧһ�����ؼ�С�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ʵ���Ũ�ȵ�������Һ�Т� NH4Al(SO4)2�� NH4Cl����CH3COONH4���� NH3��H2O�� c(NH4+)���ɴ�С��˳���Ǣ�>��>��>�� |
B�������£���0��01mol/L NH4HSO4��Һ�еμ�NaOH��Һ������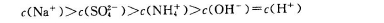 |
| C��25��ʱ��0��1mol/LCH3COOH��ҺV1 mL��0��1mol/L NaOH��ҺV2mL��ϣ���V1��V2��������Һ��pHһ��С��7 |
D�����ڷ�Ӧ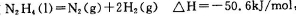 �����κ��¶��¶����Է����� �����κ��¶��¶����Է����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com