
���и���������ָ������Һ�У��ܴ�������Ĵ̡������ܴ�������Ĵ���
(1)���д���Fe3������Һ��Na����SCN����Cl����I�� (����)
(2)���д���NO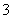 ����Һ��H����Fe2����Cl����SO
����Һ��H����Fe2����Cl����SO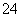 (����)
(����)
(3)�����£�pH��12����Һ��K����Cl����SO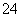 (����)
(����)
(4)c(H��)��0.1 mol��L��1����Һ��Na����NH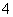 ��SO
��SO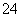 ��S2O
��S2O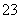 (����)
(����)
(5)ʹpH��ֽ����ɫ����Һ��Cu2����NO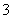 ��Fe3����SO
��Fe3����SO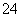 (����)
(����)
(6)�����۷�Ӧ�ų�H2����ɫ��Һ��NO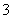 ��Al3����Na����SO
��Al3����Na����SO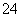 (����)
(����)
(7)ʹ��ɫʯ����ֽ��������Һ��SO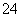 ��CO
��CO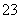 ��Na����K�� (����)
��Na����K�� (����)
(8)������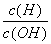 ��1��10��12����Һ��K����[Al(OH)4]����CO
��1��10��12����Һ��K����[Al(OH)4]����CO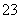 ��Na�� (����)
��Na�� (����)
(9)������Һ��Fe3����Al3����NO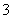 ��SO
��SO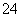 (����)
(����)
(10)ʹ���ȱ��ɫ����Һ��Mg2����K����SO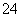 ��SO
��SO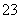 (����)
(����)
(11)c(H��)ˮ��10��12 mol��L��1����Һ��Na����K����CO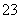 ��SO
��SO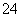 (����)
(����)
(12)ʹ��̪���ɫ����Һ��Na����Cu2����Fe2����NO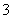 (����)
(����)
(13)0.1 mol��L��1��Na2CO3��Һ��Al3����SO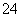 ��Cl����K�� (����)
��Cl����K�� (����)
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������˿���缫����KOH��Һ�У��ٷֱ�������ͨ�������������������γ�ȼ�ϵ�أ��õ�طŵ�ʱ���ܷ�ӦΪ��CH4+2KOH+2O2��K2CO3+3H2O������˵����������
A. ͨ�����һ��Ϊ������ͨ������һ��Ϊ����
B. �ŵ�ʱͨ��������һ��������Һ��pH����
C. �ŵ�һ��ʱ���KOH�����ʵ����������仯
D. ͨ�����һ���ĵ缫��Ӧʽ�ǣ�CH4+10OH -��8e-��CO32-+7H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
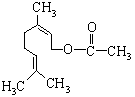
����Ȼ�����һ��ʳ�����ϣ��ṹ��ͼ��ʾ�����й��ڸ��л����˵�������¼��֣�
�ٸ��л�����һ��ͬ���칹�����ڷ��ࣻ
�ڸ��л����������ࣻ
�۸��л��ﲻ�ܷ���������Ӧ��
�ܸ��л���ķ���ʽΪC11H18O2��
��1mol���л����������1mol NaOH��Ӧ��������ȷ����
A���٢ۢ� B���ڢۢ� C���٢ܢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
OH�����ܺ�________________________________________________________��������(��������ӣ���ͬ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij��Һ�м���ϡNaOH��Һ����ʪ����ɫ��̪��ֽ�����Թܿڣ���ֽ����죬����Һ��һ��������NH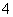 �����ж���
�����ж��� ����ȷ��Ϊʲô��
����ȷ��Ϊʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO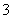 ��OH����Fe2����[Al(OH)4]����CO
��OH����Fe2����[Al(OH)4]����CO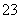 ��NH
��NH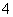 ��SO
��SO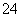 ��H����
��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | |
| �� | ȷ��M��Һ�к���Na��������K�� |
|
|
(2)����(1)�е�ʵ��ش�
NO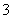 ������________��Һ�У�������_______________________________________��
������________��Һ�У�������_______________________________________��
Cl��������________��Һ�У�������________________________________________��
(3)����(1)�е�ʵ��ȷ����M��Һ�к��е�����Ϊ___________________________��
�𰸡�(1)��N��Һ�в���CO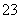 ��SO
��SO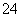 ��M��Һ��һ������CO
��M��Һ��һ������CO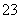 ��SO
��SO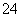
��ȡM��Һ������ɫ��Ӧ����ɫΪ��ɫ��������ɫ�ܲ����۲������ɫ��������ɫ
(2)M��N��Һ�к���H����Fe2����Al3����NH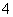 ��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO
��K��������N��ҺΪ���ԣ��ֺ���Fe2��������N��Һ�в���NO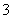
N��������Һ�ʵ�����ԭ����ȷ��Cl��������N��Һ��
(3)OH����[Al(OH)4]����CO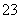 ��SO
��SO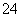 ��Na����NO
��Na����NO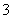
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijpH��1�Ĺ�ҵ��Һ��ֻ���ܺ������������е������֣�H����Mg2����Ba2����Cl����CO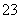 ��SO
��SO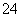 ����ȡ����100 mL��Һ��������ʵ�飺
����ȡ����100 mL��Һ��������ʵ�飺
��һ�ݼ�������AgNO3��Һ���ø������3.50 g��
�ڶ��ݼ�������BaCl2��Һ�ø������2.33 g������������ϴ�ӡ���������������䡣
��������ʵ�飬�����Ʋ���ȷ���� (����)
��һ������Mg2�����ڿ��ܴ���CO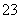 ����һ������Cl�����ܿ��ܴ���Ba2�����ݿ��ܴ���Mg2��
����һ������Cl�����ܿ��ܴ���Ba2�����ݿ��ܴ���Mg2��
A���٢� B���ڢ�
C���ۢ� D���ܢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com