
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء���֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȡ�H�ֱ�Ϊ��285.8 kJ��mol��1����283.0 kJ��mol��1�ͣ�726.5 kJ��mol��1����ش��������⣺
��1����̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________________��
��3�����ݻ�Ϊ2 L���ܱ������У���CO2��H2�ϳɼ״�(CO2+3H2  CH3OH + H2O)
CH3OH + H2O)
�������������������£������¶ȶԷ�Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��ע��T1��T2������300�棩������˵����ȷ����______������ţ�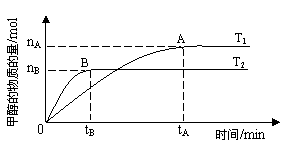
���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״���ƽ������Ϊv(CH3OH) ��nA/tA mol��L-1��min-1
�ڸ÷�Ӧ��T1ʱ��ƽ�ⳣ����T2ʱ��С
�۸÷�ӦΪ���ȷ�Ӧ
�ܴ���A��ķ�Ӧ��ϵ��T1�䵽T2���ﵽƽ��ʱn(H2)/n(CH3OH)����
��4����T1�¶�ʱ����1mol CO2��3mol H2����һ�ܱպ������У���ַ�Ӧ�ﵽƽ�����CO2ת����Ϊa,�������ڵ�ѹǿ����ʼѹǿ֮��Ϊ_________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺
��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺| nA |
| tA |
| n(H2) |
| n(CH3OH) |
| 2-a |
| 2 |
| 2-a |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
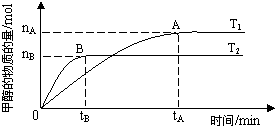 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��| nA |
| tA |
| nA |
| tA |
| a |
| 2 |
| a |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��| nA |
| tA |
| n(H2) |
| n(CH3OH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��| nA |
| tA |
| n(H2) |
| n(CH3OH) |
| 2-a |
| 2 |
| 2-a |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ�
̼�ǻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com