
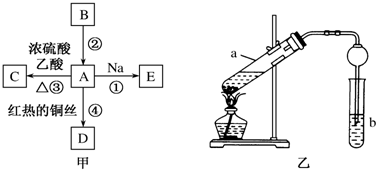
���� B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ��BΪCH2�TCH2�����ͼ��ת����֪��AΪCH3CH2OH��CH3CH2OH��Na��Ӧ����EΪCH3CH2ONa��CH3CH2OH�����ᷴӦ����CΪCH3COOCH2CH3��A������������Ӧ����DΪCH3CHO���Դ˽��1������2����
��3����a�Թ���������Ҵ�����������Ӧ��������������ˮ��
�����θ���ܳ������������⣬���νṹ�ɷ�ֹ������
��� �⣺B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ��BΪCH2�TCH2�����ͼ��ת����֪��AΪCH3CH2OH��CH3CH2OH��Na��Ӧ����EΪCH3CH2ONa��CH3CH2OH�����ᷴӦ����CΪCH3COOCH2CH3��A������������Ӧ����DΪCH3CHO��
��1��B�Ľṹ��ʽΪCH2�TCH2��A�й����ŵ�����Ϊ �ǻ���
�ʴ�Ϊ��CH2�TCH2���ǻ���
��2����Ӧ�ٵķ���ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2����
�ʴ�Ϊ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����
��3����a�Թ��е���Ҫ��ѧ��Ӧ�ķ���ʽΪCH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O����Ӧ����Ϊȡ������������Ӧ��
�ʴ�Ϊ��CH3COOH+CH3CH2OH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��ȡ������������Ӧ��
����ʵ�������θ���ܳ������������⣬��һ����Ҫ�����Ƿ�ֹ�������ʴ�Ϊ����ֹ������
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬�����л�������ʡ��ת�����л���ӦΪ���Ĺؼ������ط������ƶ������Ŀ��飬ע��BΪ��ϩ���л����ƶϵ�ͻ�ƿڣ���Ŀ�ѶȲ���


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢޢ� | B�� | �٢ڢܢ� | C�� | �ۢݢޢ� | D�� | �٢ڢۢܢݢޢߢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��� | �۵�/�� | �е�/�� | �ܶȣ�20�棩/g•cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| Ũ���� | - | 83 | 1.4 | ������ˮ |
| Ũ���� | - | 338 | 1.84 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��ͼΪijѧϰС����Ƶ���ȡ���������ĸĽ�װ�ã���ʵ��������£���ش�������⣮
��ͼΪijѧϰС����Ƶ���ȡ���������ĸĽ�װ�ã���ʵ��������£���ش�������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־��
A��ʯ���ѽ���Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
| �ܽ�� ��g/100gˮ�� | 0 | 28 | 35.7 | 4.7 | 163 |
| 40 | 40.1 | 36.4 | 26.3 | 215 | |
| 80 | 51.3 | 38 | 73 | 376 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܢۢߢ�� | B�� | �ڢۢߢݢ� | C�� | �٢ۢ�ޢ� | D�� | �ڢޢۢߢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���һԪǿ�� | B�� | Cl2���ǵ���� | C�� | CO2������� | D�� | NaHSO4����ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʱˮ��ת����Ϊ71.4% | |
| B�� | ��ʱCO2���������Ϊ20.4% | |
| C�� | ������������ͨ��5mol H2O����ﵽ��ƽ��ʱ��H2O��ת�������� | |
| D�� | ��������������5mol H2O����ﵽ��ƽ��ʱ��CO��ת�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com