
 3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺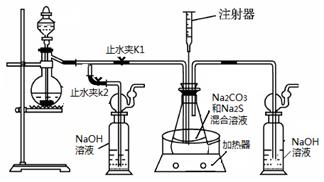
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | �۵�/�� | �е�/�� | ��ѧ���� |
| S | 112.8 | 444.6 | �� |
| S2C12 | ��77 | 137 | ��ˮ����HCl��SO2��S�� 300��������ȫ�ֽ⣻ S2C12 + C12 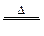 2SCl2 2SCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
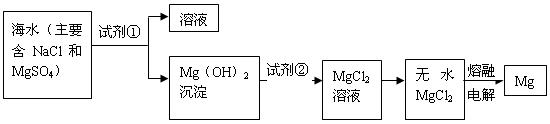
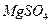 ת��Ϊ
ת��Ϊ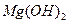 ���Լ��ٿ���ѡ�� ��Ҫʹ
���Լ��ٿ���ѡ�� ��Ҫʹ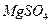 ��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��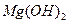 �����ķ����� ��
�����ķ����� ��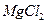 ������״̬�£�ͨ�������
������״̬�£�ͨ�������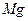 ��
��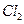 ���÷�Ӧ�Ļ�ѧ����ʽΪ��
���÷�Ӧ�Ļ�ѧ����ʽΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
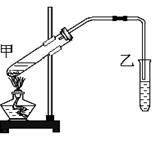
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B���������� |
| C��������ػ�������� | D������������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4Cl��ŨH2SO4��Ϲ��ȣ����ɵ������ü�ʯ�ҽ��и��� |
B��N2+3H2 �����ռ���и��� �����ռ���и��� |
| C������Ũ��ˮ�������ü�ʯ�Ҹ��� |
| D������NH4HCO3��������P2O5���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú�����ת��Ϊ��¯����ú���͡���̿�� | B��ԭ�ͷ���ɷ�������͡�ú�͡����͵� |
| C���Ӻ�ˮ�еõ�����ˮMgCl2���õ��ķ������þ | |
| D���ӵ�ˮ�еõ��⣬���þƾ���ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H4 | B��CO2 | C��CH4 | D��Cl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com