
| ʵ���� | HA | NaOH | �����Һ��pH |
| �� | [HA]��0.2 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��a |
| �� | [HA]��c1 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��7 |
| �� | [HA]��0.1 mol��L��1 | [NaOH]��0.1 mol��L��1 | pH��9 |
| �� | pH��2 | pH��12 | pH��b |


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ʹpH��ֽ�ʺ�ɫ����Һ��Na����NH ��I����NO ��I����NO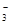 |
B��������������H2����Һ��K����Mg2����SO ��HCO ��HCO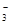 |
| C��l.0 mol��L��1 NaClO��Һ��Fe2+��K+��Iһ��Cl�� |
| D����c(H+)/c(OH��) = 1��1013����Һ��NH4+��Ca2+��C1����K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na��)��c(CH3COO��) |
| B��Ũ�Ⱦ�Ϊ0.1 mol��L��1��CH3COOH��CH3COONa��Һ�������Ϻ�c(CH3COO��)��c(CH3COOH)��2[c(H��)��c(OH��)] |
| C����pH��a�Ĵ���ϡ��ΪpH��a��1�Ĺ����У�c(CH3COOH)/c(H��)��С |
| D�������pH��a�Ĵ�����pH��b��NaOH��Һǡ���к�ʱ��a��b��14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����50 mL 1 mol��L��1�������м����ռˮ��KW���� |
| B��NH4Cl��NH3��H2O���Һ�У����߶ԶԷ���ƽ�ⶼ������������ |
| C������������HX��HY������HX��HY���������Ũ����ͬ��NaX��NaY��Һ����c(Y��)��c(X��)��c(OH��)��c(H+) |
D��������0.1 mol��L��1��HA��Һ��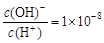 �� ��0.01 mol��L��1HA��Һ��c(H+)=1��10��4 mol��L��1 �� ��0.01 mol��L��1HA��Һ��c(H+)=1��10��4 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0��1mol/LNa2CO3��Һ��c(OH��)= 2c(H2CO3)+ c(HCO3��)+ c(H��) |
| B����ʹ��ɫʯ����Һ�ʺ�ɫ����Һ��Na����NH��I����NO���Դ������� |
| C���κ������£�pH=13������������Һ�У�c��OH����=0��1mol/L |
| D��NH4HCO3���ڹ�����NaOH��Һ�У�HCO3��+ OH��= CO32�� + H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɫ����ˮ��Һ�У�K����Ba2����Cl����MnO4�� |
| B�����д���NO3����ˮ��Һ�У�NH4����Fe2����SO42����H�� |
| C�������̪�Լ��Ժ�ɫ����Һ�У�Na����K����CO32����Br�� |
| D��ǿ������Һ�У�ClO����S2����HSO3����Na�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʹ��̪���ɫ����Һ��Fe3����Mg2����SO42����NO3�� |
| B��KNO3��������Һ��Fe2����Ca2����Al3����Cl�� |
| C�������£���ˮ�������c(H��)��1.0��10��10 mol��L��1����Һ��NH4����Na����SiO32����CO32�� |
| D��������Һ��Cu2����Fe3����NO3����MnO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ �� | ���� |
| A | 100�� 0.1 mol/LNa2SO4��ҺpH= 6.2 | H2O H+ + OH�� H+ + OH�� |
| B | 0.1 mol/L CH3COOH��pH=3 | CH3COOH CH3COO�� + H+ CH3COO�� + H+ |
| C | ����FeCl3��Һʱ���������� | Fe3+ + 3OH�� Fe(OH)3 Fe(OH)3 |
| D | ��ϡ����ϴ��BaSO4��������ʧС | BaSO4(s)  Ba2+(aq) + SO42��(aq) Ba2+(aq) + SO42��(aq) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | ����ƽ�ⳣ��(Ka��Kb) |
| CH3COOH | 1.8��10��5 |
| HNO2 | 4.6��10��4 |
| HCN | 5��10��10 |
| HClO | 3��10��8 |
| NH3��H2O | 1.8��10��5 |
| ��()���� | �ܶȻ�����(Ksp) |
| BaSO4 | 1��10��10 |
| BaCO3 | 2.6��10��9 |
| CaSO4 | 7��10��5 |
| CaCO3 | 5��10��9 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com