
| Q-393.51 |
| 395.41-Q |
| Q-393.51 |
| 395.41-Q |
 ��
��| Q-393.51 |
| 395.41-Q |
| Q-393.51 |
| 395.41-Q |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
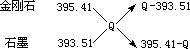
1840���˹����һϵ��ʵ����ʵ�ó����ɣ���ָ����������һ����Ӧ���Էֲ����У��������Ӧ�ķ�Ӧ���ܺ��������Ӧһ�η���ʱ�ķ�Ӧ����ͬ���������ڸ���Ӧ����ͬ���������ʱ���йط�Ӧ�ȵ���Ҫ���ɣ���Ϊ��˹���ɡ���֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��C�����ʯ��s)��O2(g)��CO2(g)����H����395.41kJ/mol��C��ʯī��s����O2(g)��CO2(g)����H����393.51kJ/mol������ʯת��ʯīʱ���Ȼ�ѧ����ʽΪ��___________���ɴ˿������ȶ���̼��ͬ��������Ϊ��____________����ȡ���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ������ʯ��ʯī�����ʵ���֮��Ϊ_____________���ú�Q�Ĵ���ʽ��ʾ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1840���˹����һϵ��ʵ����ʵ�ó����ɣ���ָ����������һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ��������18���ͷ��ֵ�һ����Ҫ���ɣ���Ϊ��˹���ɡ���֪1mol ���ʯ��ʯī�ֱ�����������ȫȼ��ʱ�ų�������Ϊ�����ʯ��395.41kJ��ʯī��393.51kJ������ʯת��ʯīʱ�����Ȼ������ȣ�_______������ֵ�� _���ɴ˿������ȶ�����_______����ȡ���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ������ʯ��ʯī�����ʵ���֮��Ϊ_____________���ú�Q�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����ѧ֪ʶ��ϵͳ���������ڶԻ�ѧ����Ľ�һ����ʶ������������й��ڻ�ѧ��Ӧ�����ۡ�
����1����ѧ�仯�����е�ƽ��״̬����ͨ���ı䷴Ӧ�����������仯���Ե���ƽ�⡢ˮ��ƽ�⡢��ѧƽ��ȸ���ƽ���ƶ��ķ�������������仯�Ĺ�ϵ��������һ�仰�����ܽ______________��
����2����ͬ��ѧ��Ӧ���еĿ����ͳ̶�ǧ������ڸ��ӵķ�Ӧ�У�Ҫ���Ƿ�Ӧ���Ⱥ�˳����֪NH4����AlO2����H2O��Al��OH��3����NH3��H2O�����е����ʵ�����NH4����Al3����H���������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ������
��1���ڶ������ӷ�Ӧ�����ӷ���ʽ��
��2�����һ�����ӷ�Ӧ�����ӷ���ʽ��
����3����ѧ��Ӧ�ĸ����Ծ����˷�Ӧ����ʽ�������ĸ����ԡ������л�ѧ��Ӧ��
8KMnO4��15Kl��17H2SO4��8MnSO4��5I2��5KIO3��9 K2SO4��17H2O
����÷�Ӧ����ʽ��I2��KIO3��ϵ������5�����ܵ���ƽϵ�����������顣������д��һ����ƽ�ĸ÷�Ӧ�Ļ�ѧ����ʽ�� ��
����4��1840���˹����һϵ��ʵ����ʵ�ó����ɣ�������һ����Ӧ���Էֲ����У��������Ӧ�ķ�Ӧ���ܺ��������Ӧһ�η���ʱ�ķ�Ӧ����ͬ���������ڸ���Ӧ����ͬ���������ʱ���йط�Ӧ�ȵ���Ҫ���ɡ���֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��C�����ʯ��s����O2��g����CO2��g������H����395��41kJ��mol��C��ʯī��s����O2��g����CO2��g������H����393��51kJ��mol������ʯת��ʯīʱ���Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com