
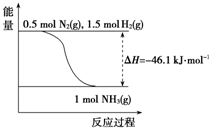
 ���ڹ�ũҵ������Ӧ�ù㷺����ѹǿΪ30MPaʱ���ϳɰ�ƽ����������NH3�����������ͼ��
���ڹ�ũҵ������Ӧ�ù㷺����ѹǿΪ30MPaʱ���ϳɰ�ƽ����������NH3�����������ͼ��| �¶�/�� | 200 | 300 | 400 | 500 | 600 |
| ������/% | 89.9 | 71.0 | 47.0 | 26.4 | 13.8 |
���� ��1������ͼд���Ȼ�ѧ����ʽ��ע�ⷴӦ�������ʵ����Ĺ�ϵ��
��2�����ݿ��淴Ӧ���в������ص�����𣬴����ı䷴Ӧ���ʲ��ı仯ѧƽ�⣬��Ӧ�ʱ䲻�䣻
��3����ѧ��Ӧ�У���ѧ�����������������γ��»�ѧ���ų����������ݷ���ʽ�����ʱ�=��Ӧ���ܼ���-��������ܼ��ܣ��Դ˼��㣻
��4�������Ȼ�ѧ����ʽ�������˹���ɵ�����ͨ���ϲ�����õ��Ȼ�ѧ����ʽ��
��� �⣺��1����ͼ��֪��$\frac{1}{2}$molN2��g����$\frac{3}{2}$molH2��g����ȫ��Ӧ����1molNH3��g���ķ�Ӧ��Ϊ-46.1kJ/mol��
���Ժϳɰ����Ȼ�ѧ��Ӧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92.2kJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92.2 kJ•mol-1��
��2����Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת����3mol H2��1mol N2��ֲ��뷴ӦҲ����������2molNH3���ʷų�������С��92KJ�������������ı䷴Ӧ���ʲ��ı仯ѧƽ�⣬��Ӧ�ʱ䲻�䣻
�ʴ�Ϊ��С�ڣ��÷�Ӧ�ǿ��淴Ӧ����Ӧ����ȫ��ת��Ϊ��������䣻
��3����֪���ֱ��ƻ�1mol N��N����1mol H-H����Ҫ���յ�����Ϊ��946kJ��436kJ�����ƻ�1mol N-H����Ҫ���յ�����Ϊx��N2��g��+3H2��g��?2NH3��g����H=-92.2 kJ•mol-1���ʱ�=��Ӧ���ܼ���-��������ܼ��ܣ�946kJ/mol+3��436kJ/mol-6x=92.2KJ/mol��x=391KJ/mol���ƻ�1mol N-H����Ҫ���յ�����Ϊ��391KJ��
�ʴ�Ϊ��391��
��4������N2��g��+O2��g��=2NO2��g����H1=+67.7kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g����H2=-534kJ/mol
���ݸ�˹���ɣ��ڡ�2-�ٵõ���2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=-1135.7KJ/mol��1mol N2H4��NO2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ��N2H4��g��+NO2��g���T$\frac{3}{2}$N2��g��+2H2O��g����H=-567.85 kJ•mol-1��
�ʴ�Ϊ��N2H4��g��+NO2��g���T$\frac{3}{2}$N2��g��+2H2O��g����H=-567.85 kJ•mol-1��
���� ���⿼�����Ȼ�ѧ����ʽ����д������˹���ɵļ���Ӧ�ã����ݼ��ܼ����ʱ䣬ע����淴Ӧ���ܽ��г��ף���Ŀ�ϼ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ħ��������98g | B�� | 1mol N2������Ϊ28g/mol | ||
| C�� | ����٤�������÷���ΪNA��ʾ | D�� | 1mol������������16g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ܵ��磬�������ǵ���� | |
| B�� | ϡ�����ܵ��磬���������ǵ���� | |
| C�� | ������Һ�ܵ��磬���������ǵ���� | |
| D�� | Na2O����ˮ�ܵ��磬��Na2O���ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
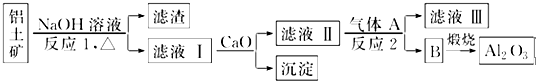
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȴ�������ˮ | B�� | �ȴ�����ˮ | C�� | �ȴ��������� | D�� | �ȴ���������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �Ĵ� | C�� | �崦 | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X�����γɻ�ѧʽΪHXO3���� | |
| B�� | X������ijЩ����Ԫ���γɻ����� | |
| C�� | Xԭ�ӵ������������ͺ˵�����϶�Ϊ���� | |
| D�� | X�������γɻ�ѧʽΪKXO4���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com