
ΓΨΧβΡΩΓΩΘ®1Θ©“―÷ΣΖ¥”Π![]() ΒΡ
ΒΡ![]() Θ§
Θ§![]() ΓΔ
ΓΔ![]() Ζ÷Ή”÷–Μ·―ßΦϋΕœΝ― ±Ζ÷±π–η“ΣΈϋ ’436 kJΓΔ151 kJΒΡΡήΝΩΘ§‘ρ1 mol HI(g)Ζ÷Ή”÷–Μ·―ßΦϋΕœΝ― ±–ηΈϋ ’ΒΡΡήΝΩΈΣ_____kJΓΘ
Ζ÷Ή”÷–Μ·―ßΦϋΕœΝ― ±Ζ÷±π–η“ΣΈϋ ’436 kJΓΔ151 kJΒΡΡήΝΩΘ§‘ρ1 mol HI(g)Ζ÷Ή”÷–Μ·―ßΦϋΕœΝ― ±–ηΈϋ ’ΒΡΡήΝΩΈΣ_____kJΓΘ
Θ®2Θ©Ε‘”Ύ“ΜΑψΒΡΜ·―ßΖ¥”ΠaA+bBΓζPΘ§Ζ¥”ΠΈο≈®Ε»ΚΆΖ¥”ΠΥΌ¬ ÷°Φδ¥φ‘Ύ“‘œ¬ΙΊœΒΘΚ vΘ®ΒΞΈΜmolΓΛL-1ΓΛmin-1Θ©ΘΫkΓΝc(A)x ΓΝc(B)yΘ§k≥ΤΈΣΥΌ¬ ≥Θ ΐΘ§x≥ΤΈΣΖ¥”ΠΈοAΒΡΦΕ ΐΘ§y≥ΤΈΣΖ¥”ΠΈοBΒΡΦΕ ΐΘ§x+y≥ΤΈΣΖ¥”ΠΒΡΉήΦΕ ΐΓΘ
ΔΌ Ε‘”ΎΖ¥”ΠH2(g)ΘΪI2(g)Γζ2HI(g)Θ§ΥΌ¬ ±μ¥ο ΫΘΚvΘΫkΓΝc(H2)ΓΝc(I2)Θ§‘ρΖ¥”ΠΉήΦΕ ΐΈΣ________ΦΕΓΘ
ΔΎ H2O2Ζ÷Ϋβ≥…Υ°ΚΆ―θΤχΒΡΖ¥”Π «“ΜΦΕΖ¥”ΠΘ§Ζ¥”ΠΥΌ¬ ≥Θ ΐkΈΣ0.0410 min-1Θ§‘ρΙΐ―θΜ·«βΖ÷Ϋβ“ΜΑκΒΡ ±Φδ «__________minΓΘΘ®ΫαΙϊ±ΘΝτ3ΈΜ”––ß ΐΉ÷Θ©Θ®“―÷ΣΘΚ“ΜΦΕΖ¥”ΠΈο÷ ≈®Ε»c=c0e-ktΘ§c0ΈΣ≥θ Φ≈®Ε»Θ§ln2=0.693Θ©
Θ®3Θ©ΫΪΒ»Έο÷ ΒΡΝΩΒΡI2ΚΆH2÷Ο”Ύ‘Λœ»≥ι’φΩ’ΒΡΧΊ÷Τ1LΟή±’»ίΤς÷–Θ§Φ”»»ΒΫ1500 KΘ§Τπ ΦΉή―Ι«ΩΈΣ416kPaΘΜΧεœΒ¥οΤΫΚβΘ§Ήή―Ι«ΩΈΣ456kPaΓΘΧεœΒ÷–¥φ‘Ύ»γœ¬Ζ¥”ΠΙΊœΒΘΚ
ΔΌI2(g)2I(g) Kp1 = 200 ΠΛH1
ΔΎI2(g)+ H2(g)2HI(g) Kp2 ΠΛH2
ΔέHI(g)I(g)+ H(g) Kp3 ΠΛH3
ΔήH2(g)2H(g) Kp4 ΠΛH4
Θ®“―÷ΣKp3ΓΔ Kp4÷ΒΚή–ΓΘ§ΔέΓΔΔήΖ¥”ΠΚω¬‘ΘΜKpΈΣ“‘Ζ÷―Ι±μ ΨΒΡΤΫΚβ≥Θ ΐΘ§“‘œ¬ΦΤΥψΫαΙϊΨυ±ΘΝτ2ΈΜ”––ß ΐΉ÷Θ©
ΔΌΠΛH2=_____________ΓΘΘ®”ΟΚ§ΠΛH1ΓΔΠΛH3ΓΔΠΛH4ΒΡ ΫΉ”±μ ΨΘ©
ΔΎ1500KΤΫΚβΧεœΒ÷–I(g)ΓΔH2(g)Ζ÷―ΙΖ÷±πΈΣ______kPaΓΔ_______kPaΓΔKp2=______ΓΘ
ΓΨ¥πΑΗΓΩ299 Εΰ 16.9 ΠΛH1+ΠΛH4-2ΠΛH3 80 72 32
ΓΨΫβΈωΓΩ
(1)άϊ”ΟΖ¥”ΠΈϋ ’ΒΡΉήΡήΝΩ”κ ΆΖ≈ΒΡΉήΡήΝΩΒΡ≤ν÷ΒΒ»”Ύλ ±δΫχ––ΦΤΥψΘΜ
(2)ΔΌΗυΨίΥΌ¬ ≥Θ ΐ±μ¥ο ΫΒΟ≥ωxΘ§yΒΡ÷ΒΘ§ΉήΦΕ ΐΒ»”Ύx+yΘΜ
ΔΎΗυΨίΥυΗχΙΪ ΫΚΆΧβ÷–“―÷ΣΧθΦΰΘ§ΫαΚœΉ‘»ΜΕ‘ ΐΒΡ±μ ΨΖΫΖ®Ϋχ––ΦΤΥψ ±ΦδtΘΜ
(3)ΔΌάϊ”ΟΗ«ΥΙΕ®¬…ΦΤΥψΠΛH2ΘΜ
ΔΎΝ–»ΐΕΈ ΫΘ§άϊ”ΟΤΫΚβ ±ΒΡ―Ι«Ω±μ ΨΜ·―ßΤΫΚβ≥Θ ΐΘ§ΦΤΥψ≥ωΗςΈο÷ ΒΡΖ÷―ΙΚΆKp2ΓΘ
(1)λ ±δΒ»”ΎΖ¥”ΠΈϋ ’ΒΡΉήΡήΝΩ”κ ΆΖ≈ΒΡΉήΡήΝΩΒΡ≤νΘ§…η1molHI(g)Ζ÷Ή”÷–Μ·―ßΦϋΕœΝ― ±–ηΈϋ ’ΒΡΡήΝΩΈΣxkJΘ§‘ρΘΚ2xkJ436kJ151kJ=11kJΘ§ΫβΒΟx=299ΘΜ
(2)ΔΌΗυΨίΧβ“βΥΌ¬ ≥Θ ΐ±μ¥ο Ϋ÷–ΗςΈοάμΝΩΖϊΚ≈ΒΡΚ§“εΘ§Ζ¥”ΠH2(g)ΘΪI2(g)Γζ2HI(g)Θ§ΥΌ¬ ±μ¥ο ΫΘΚvΘΫkΓΝc(H2)ΓΝc(I2)Θ§‘ρx=1Θ§y=1Θ§ΗΟΉήΦΕ ΐ=x+y=2ΘΜ
ΔΎΗυΨίΧβ“βΘ§Ιΐ―θΜ·«βΖ÷Ϋβ“ΜΑκΘ§‘ρΖ÷Ϋβ“ΜΑκ ±ΒΡ≈®Ε»c=![]() c0ΓΘ‘ρ
c0ΓΘ‘ρ![]() ΘΜ“―÷Σc=c0e-ktΘ§‘ρ
ΘΜ“―÷Σc=c0e-ktΘ§‘ρ![]() = e-ktΘ§Β» ΫΝΫ±Ώ»ΓΉ‘»ΜΕ‘ ΐΘ§ln
= e-ktΘ§Β» ΫΝΫ±Ώ»ΓΉ‘»ΜΕ‘ ΐΘ§ln![]() = ln e-kt=- ktΘ§ln2=ktΘ§k=0.0410 min-1Θ§ln2=0.693Θ§Ι t=
= ln e-kt=- ktΘ§ln2=ktΘ§k=0.0410 min-1Θ§ln2=0.693Θ§Ι t=![]() =16.9minΘΜ
=16.9minΘΜ
(3)ΔΌ“―÷ΣΘΚΔΌI2(g)2I(g)ΠΛH1Θ§ΔΎI2(g)+ H2(g)2HI(g)ΠΛH2Θ§ΔέHI(g)I(g)+ H(g)ΠΛH3Θ§ΔήH2(g)2H(g)ΠΛH4ΘΜΗυΨίΗ«ΥΙΕ®¬…Ω…÷ΣΘ§Ζ¥”ΠΘΚΔΎ=ΔΌ+2ΓΝΔέ+ΔήΘ§Ω…ΒΟΠΛH2=ΠΛH1+ΠΛH4-2ΠΛH3ΘΜ
ΔΎΫΪΒ»Έο÷ ΒΡΝΩΒΡI2ΚΆH2÷Ο”ΎΟή±’»ίΤς÷–Θ§Τπ ΦΉή―Ι«ΩΈΣ416kPaΘ§Ι I2ΚΆH2ΒΡΤπ ΦΖ÷―ΙΖ÷±πΈΣ208 kPaΘ§”…ΧεœΒ¥οΤΫΚβΘ§Ήή―Ι«ΩΈΣ456kPaΘ§‘ρΟή±’»ίΤς÷–ΤχΧεΧεΜΐ‘ω¥σΘ§“―÷ΣKp3ΓΔ Kp4÷ΒΚή–ΓΘ§‘ρ»ίΤς÷–”–ΝΫ≤ΫΖ¥”ΠΖΔ…ζΘ§Φ¥ΔΌI2(g)2I(g)Kp1 = 200Θ§ΔΎI2(g)+ H2(g)2HI(g)Θ§άϊ”Ο»ΐΕΈ ΫΘ§…ηI2ΚΆH2ΒΡΉΣΜ·Ζ÷―ΙΖ÷±πΈΣxΘ§

…ηI2(g)ΒΡΉΣΜ·Ζ÷―ΙΈΣyΘ§‘ρΝ–ΓΑ»ΐΕΈ ΫΓ±ΘΚ

“―÷ΣKp1 = 200 ΧεœΒ¥οΤΫΚβΘ§Ήή―Ι«ΩΈΣ456kPaΘ§Ζ¥”ΠΔΌΒΡ―Ι«Ω±μ ΨΒΡΤΫΚβ≥Θ ΐKp1 = =200Θ§208-x+2x +2y+208-x-y=456Θ§ΫβΒΟy=40Θ§x=136Θ§1500KΤΫΚβΧεœΒ÷–IΘ®gΘ©ΓΔH2Θ®gΘ©Ζ÷―ΙΖ÷±πΈΣ2y=80kPaΘ§208-x=72kPaΘ§Kp2=
=200Θ§208-x+2x +2y+208-x-y=456Θ§ΫβΒΟy=40Θ§x=136Θ§1500KΤΫΚβΧεœΒ÷–IΘ®gΘ©ΓΔH2Θ®gΘ©Ζ÷―ΙΖ÷±πΈΣ2y=80kPaΘ§208-x=72kPaΘ§Kp2=  =32ΓΘ
=32ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥Ά≠νήΩσ ·÷ς“ΣΚ§”–CoOOHΓΔCoCO3ΓΔCu2(OH)2CO3ΚΆSiO2Θ§Τδ÷–ΜΙΚ§”–“ΜΕ®ΝΩΒΡFe2O3ΓΔMgOΚΆCaOΒ»ΓΘ”…ΗΟΩσ ·÷Τ±ΗCo2O3ΒΡ≤ΩΖ÷ΙΛ“’Ιΐ≥Χ»γœ¬ΘΚ
IΘ°ΫΪΖέΥιΒΡΩσ ·”ΟΙΐΝΩΒΡœΓH2SO4ΚΆNa2SO3»ή“ΚΫΰ≈ίΘ§Ιΐ¬ΥΘ§Ζ÷άκ≥ΐ»Ξ≥ΝΒμaΓΘ
IIΘ°Ϋΰ≥ω“Κ≥ΐ»ΞΚ§Ά≠ΒΡΜ·ΚœΈοΚσΘ§œρ»ή“Κ÷–œ»Φ”»κNaClO3»ή“ΚΘ§‘ΌΦ”»κ“ΜΕ®≈®Ε»ΒΡNa2CO3»ή“ΚΘ§Ιΐ¬ΥΘ§Ζ÷άκ≥ΐ»Ξ≥ΝΒμb[÷ς“Σ≥…Ζ÷ «Na2Fe6(SO4)4(OH)12]ΓΘ
IIIΘ°œρ…œ ω¬Υ“Κ÷–Φ”»κΉψΝΩNaF»ή“ΚΘ§Ιΐ¬ΥΘ§Ζ÷άκ≥ΐ»Ξ≥ΝΒμcΓΘ
IVΘ°III÷–¬Υ“ΚΦ”»κ≈®Na2CO3»ή“ΚΘ§ΜώΒΟCoCO3≥ΝΒμΓΘ
VΘ°ΫΪCoCO3»ήΫβ‘Ύ―ΈΥα÷–Θ§‘ΌΦ”»κ(NH4)2C2O4»ή“ΚΘ§≤ζ…ζ CoC2O4ΓΛ2H2O≥ΝΒμΓΘΖ÷άκ≥ω≥ΝΒμΘ§ΫΪΤδ‘Ύ400Γφ~600Γφ λ―…’Θ§Φ¥ΒΟΒΫCo2O3ΓΘ
«κΜΊ¥πΘΚ
Θ®1Θ©I ÷–Θ§≥ΝΒμaΒΡ≥…Ζ÷ «_____Θ§œΓΝρΥα»ήΫβCoCO3ΒΡΜ·―ßΖΫ≥Χ Ϋ «_____Θ§ Φ”»κNa2SO3»ή“ΚΒΡ÷ς“ΣΉς”Ο «_________ΓΘ
Θ®2Θ©ΗυΨίΆΦ1ΓΔΆΦ2

ΔΌΩσ ·ΖέΡ©Ϋΰ≈ίΒΡ “ΥΧθΦΰ”Π «ΘΚΈ¬Ε»_____ΓΔpH_____ΓΘ
ΔΎΆΦ2÷–Ά≠ΓΔνήΫΰ≥ω¬ œ¬ΫΒΒΡΩ…Ρή‘≠“ρ «_____ΓΘ
Θ®3Θ©II÷–Θ§Ϋΰ≥ω“Κ÷–ΒΡΫπ τάκΉ””κNaClO3Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚClO![]() +_____+_____== Cl- +_____+ _____
+_____+_____== Cl- +_____+ _____
Θ®4Θ©I÷–Θ§Φλ―ιΧζ‘ΣΥΊΆξ»Ϊ≥ΐ»ΞΒΡ ‘ΦΝ «_____Θ§ Β―ιœ÷œσ «_____ΓΘ
Θ®5Θ©I÷–Θ§≥ΝΒμcΒΡ≥…Ζ÷ «CaF2ΓΔ_____Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®6Θ©V÷–Θ§Φ”»κNa2CO3ΒΡΉς”Ο «_____ΓΘ
Θ®7Θ©V÷–Θ§Ζ÷άκ≥ω¥ΩΨΜΒΡCoC2O4ΓΛ2H2OΒΡ≤ΌΉς «_____ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΕκΟΦΫπΕΞ…ψ…μ―¬”÷≥Τ…α…μ―¬Θ§“ρ≥Θœ÷ΖπΙβΕχΒΟΟϊΓΘΓΑΖπΙβΓ±“ρ…ψ»κ…μ÷°”Αœώ”ΎΤδ÷–Θ§Υλ≥ΤΓΑ…ψ…μΙβΓ±Θ§ΈΣΕκΟΦ ΛΨΑ÷°“ΜΓΘ…ψ…ζ―¬œ¬ΆΝ»ά÷–ΗΜΚ§ΝΉΩσΘ§Υυ“‘‘ΎΈό‘¬ΒΡΚΎ“ΙΩ…ΦϊΒΫ―¬œ¬”ΪΙβΈό ΐΓΘ
(1)ΓΑ”ΪΙβΓ±÷ς“Σ≥…Ζ÷ «PH3Θ§ΤδΫαΙΙ ΫΈΣ___________Θ§œ¬Ν–”–ΙΊPH3ΒΡΥΒΖ®¥μΈσΒΡ «___________ΓΘ
a.PH3Ζ÷Ή” «ΦΪ–‘Ζ÷Ή”
b.PH3Ζ÷Ή”Έ»Ε®–‘ΒΆ”ΎNH3Ζ÷Ή”Θ§“ρΈΣNΘ≠HΦϋΦϋΡήΗΏ
c.“ΜΗωPH3Ζ÷Ή”÷–Θ§P‘≠Ή”ΚΥΆβ”–“ΜΕ‘Ι¬ΒγΉ”Ε‘
d.PH3Ζ–ΒψΒΆ”ΎNH3Ζ–ΒψΘ§“ρΈΣPΘ≠HΦϋΦϋΡήΒΆ
(2)ΓΑ”ΪΙβΓ±≤ζ…ζΒΡ‘≠άμ «Ca3P2‘Ύ≥± ΣΒΡΩ’Τχ÷–ΨγΝ“Ζ¥”ΠΘ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ____________________ΓΘ
(3)“―÷Σœ¬Ν–ΦϋΡή ΐΨίΦΑP4(ΑΉΝΉ)Ζ÷Ή”ΫαΙΙΘΚ
Μ·―ßΦϋ | PΘ≠P | HΘ≠H | PΘ≠H | ΑΉΝΉΖ÷Ή”ΫαΙΙ |
ΦϋΡή/(kJΓΛmol-1) | 213 | 436 | 322 |
|
‘ρΖ¥”Π4PH3(g)![]() P4(g)ΘΪ6H2(g) ΓςHΘΫ___________ kJΓΛ molΘ≠1ΓΘ
P4(g)ΘΪ6H2(g) ΓςHΘΫ___________ kJΓΛ molΘ≠1ΓΘ
(4)Ρ≥Έ¬Ε»œ¬Θ§œρ»ίΜΐΈΣ2LΒΡΟή±’»ίΤς÷–Ά®»κ2 mol PH3ΖΔ…ζ(3)÷–Ζ¥”ΠΘ§5minΚσΖ¥”Π¥οΤΫΚβΘ§≤βΒΟ¥Υ ±H2ΒΡΈο÷ ΒΡΝΩΈΣ1.5 molΘ§‘ρ”ΟPH3±μ ΨΒΡ’βΕΈ ±ΦδΡΎΒΡΜ·―ßΖ¥”ΠΥΌ¬ v(PH3)ΘΫ__________ΘΜœ¬Ν–ΥΒΖ®Ρή±μΟςΗΟΖ¥”Π¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «___________ΓΘ
A.ΜλΚœΤχΧεΒΡΟήΕ»≤Μ±δ B.6v(PH3)ΘΫ4v(H2)
C.c(PH3)ΘΚc(P4)ΘΚc(H2)ΘΫ4ΘΚ1ΘΚ6 D.ΜλΚœΤχΧεΒΡ―Ι«Ω≤Μ±δ
(5)PH3”–ΕΨΘ§ΑΉΝΉΙΛ≥ß≥Θ”ΟCu2ΘΪΓΔPd2ΘΪ“ΚœύΆ―≥ΐPH3ΘΚPH3ΘΪ2O2![]() H3PO4Θ§ΤδΥϊΧθΦΰœύΆ§ ±Θ§»ήΫβ‘Ύ»ή“Κ÷–O2ΒΡΧεΜΐΖ÷ ΐ”κPH3ΒΡΨΜΜ·–߬ ”κ ±ΦδΒΡΙΊœΒ»γΆΦΥυ ΨΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
H3PO4Θ§ΤδΥϊΧθΦΰœύΆ§ ±Θ§»ήΫβ‘Ύ»ή“Κ÷–O2ΒΡΧεΜΐΖ÷ ΐ”κPH3ΒΡΨΜΜ·–߬ ”κ ±ΦδΒΡΙΊœΒ»γΆΦΥυ ΨΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ

(I)”…ΆΦΩ…÷ΣΘ§ΗΜ―θ”–άϊ”Ύ____________(―ΓΧνΓΑ―”≥ΛΓ±ΜρΓΑΥθΕΧΓ±)¥ΏΜ·Ής”ΟΒΡ≥÷–χ ±ΦδΓΘ
(Δρ)ΥφΉ≈Ζ¥”ΠΫχ––Θ§PH3ΒΡΨΜΜ·–߬ Φ±ΨγΫΒΒΆΒΡ‘≠“ρΩ…ΡήΈΣ_________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΙΛ“Β…œ≥Θ”ΟΧζ÷ »ίΤς ΔΉΑάδΒΡ≈®ΝρΥαΓΘΒΪΡ≥–Υ»Λ–ΓΉιΒΡΆ§―ßΖΔœ÷ΫΪ“ΜΕ®ΝΩΒΡ…ζΧζ”κ≈®ΝρΥαΦ”»» ±Θ§Ιέ≤λΒΫΙΧΧεΡήΆξ»Ϊ»ήΫβΘ§≤Δ≤ζ…ζ¥σΝΩΤχΧεΓΘΈΣ¥ΥΥϊΟ«Ϋχ––ΝΥ»γœ¬ΧΫΨΩ Β―ιΓΘ
[ΧΫΨΩ“Μ]≥Τ»ΓΧζΕΛ(ΧΦΥΊΗ÷)6.0gΖ≈»κ15.0 mL≈®ΝρΥα÷–Θ§Φ”»»Θ§≥δΖ÷Ζ¥”ΠΚσΒΟΒΫ»ή“ΚXΘ§≤Δ ’Φ·ΒΫΤχΧεYΓΘ
(1)(I)ΦΉΆ§―ß»œΈΣX÷–≥ΐFe3ΘΪΆβΜΙΩ…ΡήΚ§”–Fe2ΘΪΓΘ»τ“Σ≈–Εœ»ή“ΚX÷– «ΖώΚ§”–Fe2ΘΪΘ§Ω…“‘―Γ”Ο___________ΓΘ
a.KSCN»ή“ΚΚΆ¬»Υ° b.K3[Fe(CN)6]»ή“Κ c.≈®Α±Υ° d.Υα–‘KMnO4»ή“Κ
(Δρ)““Ά§―ßΫΪ336 mL(±ξΉΦΉ¥Ωω)ΤχΧεYΆ®»κΉψΝΩδεΥ°÷–Θ§ΖΔœ÷»ή“Κ―’…Ϊ±δ«≥Θ§ ‘”ΟΜ·―ßΖΫ≥Χ ΫΫβ ΆδεΥ°―’…Ϊ±δ«≥ΒΡ‘≠“ρ___________Θ§»ΜΚσœρΖ¥”ΠΚσΒΡ»ή“Κ÷–Φ”»κΉψΝΩBaCl2»ή“ΚΘ§Ψ≠ Β±≤ΌΉςΒΟΗ…‘οΙΧΧε2.33 gΓΘ”…¥ΥΆΤ÷ΣΤχΧεY÷–SO2ΒΡΧεΜΐΖ÷ ΐΈΣ___________ΓΘ(ΫαΙϊ±ΘΝτ“ΜΈΜ–Γ ΐ)ΓΘ
[ΧΫΨΩΕΰ]ΦΉ““ΝΫΆ§―ß»œΈΣΤχΧεY÷–≥ΐSO2ΆβΘ§ΜΙΩ…ΡήΚ§”–H2ΚΆCO2ΓΘΈΣ¥Υ…ηΦΤ»γΆΦ Β―ιΉΑ÷Ο(ΆΦ÷–Φ–≥÷“«Τς Γ¬‘)Ϋχ––―ι÷ΛΓΘ

(2)Φρ ωΗΟ Β―ιΡή≤ζ…ζ…ΌΝΩH2ΒΡ‘≠“ρ___________(”ΟΜ·―ß”Ο”οΫαΚœ…ΌΝΩΈΡΉ÷±μ ω)ΓΘ
(3)ΉΑ÷ΟB÷– ‘ΦΝΒΡΉς”Ο «___________Θ§ΉΑ÷ΟFΒΡΉς”Ο «___________ΓΘ
(4)ΈΣΝΥΫχ“Μ≤Ϋ»Ζ»œCO2ΒΡ¥φ‘ΎΘ§–η‘Ύ…œ ωΉΑ÷Ο÷–ΧμΦ”M”Ύ___________(―ΓΧν–ρΚ≈)Θ§M÷–Υυ ΔΉΑΒΡ ‘ΦΝΩ…“‘ «___________ΓΘ
a.AΓΪB÷°Φδ b.BΓΪC÷°Φδ c.CΓΪD÷°Φδ d.EΓΪF÷°Φδ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒγ…χΈωΖ®¥Πάμ≥χΖΩά§ΜχΖΔΫΆ“ΚΘ§Ά§ ±ΒΟΒΫ»ιΥαΒΡ‘≠άμ»γΆΦΥυ ΨΘ®ΆΦ÷–ΓΑHAΓ±±μ Ψ»ιΥαΖ÷Ή”Θ§»ιΥαΒΡΡΠΕϊ÷ ΝΩΈΣ90g/moLΘΜΓΑAΘ≠Γ±±μ Ψ»ιΥαΗυάκΉ”Θ©ΓΘ‘ρœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «

A.ΫΜΜΜΡΛ IΈΣ÷Μ‘ –μ―τάκΉ”ΆΗΙΐΒΡ―τάκΉ”ΫΜΜΜΡΛ
B.―τΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣΘΚ2H2OΘ≠4eΘ≠ΘΫO2ΓϋΘΪ4H+
C.ΒγΫβΙΐ≥Χ÷–≤…»Γ“ΜΕ®ΒΡ¥κ ©Ω…ΩΊ÷Τ“θΦΪ “ΒΡpH‘ΦΈΣ6ΓΪ8Θ§¥Υ ±Ϋχ»κ≈®Υθ “ΒΡOHΘ≠Ω…Κω¬‘≤ΜΦΤΓΘ…η200mL 20g/L»ιΥα»ή“ΚΆ®Βγ“ΜΕΈ ±ΦδΚσ“θΦΪ…œ≤ζ…ζΒΡH2‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐ‘ΦΈΣ6.72LΘ§‘ρΗΟ»ή“Κ≈®Ε»…œ…ΐΈΣ155g/LΘ®»ή“ΚΧεΜΐ±δΜ·Κω¬‘≤ΜΦΤΘ©
D.≈®Υθ “ΡΎ»ή“ΚΨ≠ΙΐΒγΫβΚσpHΫΒΒΆ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΟάΆ–¬εΕϊΩ…”Ο”Ύ÷ΈΝΤΗΏ―Σ―ΙΦΑ–ΡΫ Ά¥Θ§Ρ≥Κœ≥…¬ΖœΏ»γœ¬ΘΚ

ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ωC÷–Ρή‘ΎNaOH»ή“ΚάοΖΔ…ζΖ¥”ΠΒΡΙΌΡήΆ≈ΒΡΟϊ≥Τ______ΓΘ
Θ®2Θ©AΓζBΚΆCΓζDΒΡΖ¥”Πάύ–ΆΖ÷±π «___________ΓΔ____________Θ§HΒΡΖ÷Ή” ΫΈΣ______ΓΘ
Θ®3Θ©Ζ¥”ΠEΓζFΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______ΓΘ
Θ®4Θ© ‘ΦΝXΒΡΖ÷Ή” ΫΈΣC3H5OClΘ§‘ρXΒΡΫαΙΙΦρ ΫΈΣ______ΓΘ
Θ®5Θ©BΒΡΆ§Ζ÷“λΙΙΧε÷–Θ§–¥≥ωΖϊΚœ“‘œ¬ΧθΦΰΘΚΔΌΚ§”–±ΫΜΖΘΜΔΎΡήΖΔ…ζ“χΨΒΖ¥”ΠΘΜΔέ±ΫΜΖ…œ÷Μ”–“ΜΗω»Γ¥ζΜυ«“ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΒΡ”–ΜζΈοΒΡΫαΙΙΦρ Ϋ____________ΓΘ
Θ®6Θ©4Θ≠ή–Μυ±ΫΖ”Θ®![]() Θ© «“Μ÷÷“©Έο÷–ΦδΧεΘ§«κ…ηΦΤ“‘±ΫΦΉΥαΚΆ±ΫΖ”ΈΣ‘≠Νœ÷Τ±Η4Θ≠ή–Μυ±ΫΖ”ΒΡΚœ≥…¬ΖœΏΘΚ____________Θ®ΈόΜζ ‘ΦΝ»Έ”ΟΘ©ΓΘ
Θ© «“Μ÷÷“©Έο÷–ΦδΧεΘ§«κ…ηΦΤ“‘±ΫΦΉΥαΚΆ±ΫΖ”ΈΣ‘≠Νœ÷Τ±Η4Θ≠ή–Μυ±ΫΖ”ΒΡΚœ≥…¬ΖœΏΘΚ____________Θ®ΈόΜζ ‘ΦΝ»Έ”ΟΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν––π ω÷–’ΐ»ΖΒΡ «( )
A.≥ΘΈ¬œ¬Θ§![]() ΒΡ«β―θΜ·ΡΤ»ή“Κ÷–Φ”»κ10mL
ΒΡ«β―θΜ·ΡΤ»ή“Κ÷–Φ”»κ10mL ![]() ΒΡHAΘ§ΥυΒΟ»ή“Κ
ΒΡHAΘ§ΥυΒΟ»ή“Κ![]()
B.![]() ±Θ§
±Θ§![]() ”κ
”κ![]() ΒΡ¬»Μ·οß»ή“ΚΒΡpH«Α’Ώ¥σ
ΒΡ¬»Μ·οß»ή“ΚΒΡpH«Α’Ώ¥σ
C. “Έ¬ ±≈®Ε»ΨυΈΣ![]() ΒΡ
ΒΡ![]() ΚΆ
ΚΆ![]() ΒΡΜλΚœ“ΚΘ§pHΈΣ10Θ§‘ρ
ΒΡΜλΚœ“ΚΘ§pHΈΣ10Θ§‘ρ![]()
D.![]() »ή“ΚΘΚ
»ή“ΚΘΚ![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§œ¬Ν–»ή“ΚΒΡάκΉ”≈®Ε»ΙΊœΒ Ϋ’ΐ»ΖΒΡ «
A. pH=5ΒΡH2S»ή“Κ÷–Θ§c(H+)= c(HSΘ≠)=1ΓΝ10ΓΣ5molΓΛLΓΣ1
B. pH=aΒΡΑ±Υ°»ή“ΚΘ§œΓ Ά10±ΕΚσΘ§ΤδpH=bΘ§‘ρa=b+1
C. pH=2ΒΡH2C2O4»ή“Κ”κpH=12ΒΡNaOH»ή“Κ»Έ“β±»άΐΜλΚœΘΚc(NaΘΪ)+ c(HΘΪ)= c(OHΘ≠)+c( HC2O4Θ≠)
D. pHœύΆ§ΒΡΔΌCH3COO NaΔΎNaHCO3ΔέNaClO»ΐ÷÷»ή“ΚΒΡc(NaΘΪ)ΘΚΔΌΘΨΔΎΘΨΔέ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΒγΕΤΙΛ“Β…œΘ§ΈΣΝΥΧαΗΏΕΤ–ΩΒΡ–ßΙϊΘ§Ά®≥Θ≤…”ΟZn(CN)42Θ≠»ή“Κ¥ζΧφZn2+»ή“ΚΫχ––ΒγΫβΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)‘ΣΥΊ–Ω‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷ΟΈΣ_____________Θ§ΜυΧ§ZnΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ_____________ΓΘ
(2)Zn(CN) 42Θ≠ΥυΚ§‘ΣΥΊ÷–Θ§ΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊ «_____________Θ§Zn(CN) 42Θ≠÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά”–Π“ΦϋΚΆ_____________ΓΘ
(3)CNΘ≠÷–CΒΡ‘”Μ·άύ–ΆΈΣ_____________Θ§”κCNΘ≠ΜΞΈΣΒ»ΒγΉ”ΧεΒΡΒΞ÷ ΈΣ_____________ΓΘ
(4)H2CO3”κHNO3ΒΡΥα–‘œύ≤νΫœ¥σΘ§«κΫβ ΆΤδ‘≠“ρ_____________ΓΘ
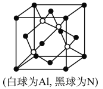
(5)NΚΆAlΩ…Ήι≥…“Μ÷÷–¬–ΆΑκΒΦΧε≤ΡΝœAlNΘΜAlNΨΏ”–ΡΆΗΏΈ¬Θ§ΡΆΡΞ–‘ΡήΓΘΤδΨßΧεάύ–ΆΈΣ_____________Θ§ΤδΨßΧεΫαΙΙ»γΆΦΘ§“―÷ΣΨßΑϊ±Ώ≥ΛΈΣapmΘ§‘ρAlNΒΡΟήΕ»ΈΣ_____________(”ΟΚ§aΓΔNAΒΡ¥ζ ΐ Ϋ±μ Ψ)g/cm3ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com