
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
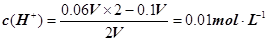 ����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4 mol��L�C1���������Ϻ�Ļ����Һ��
����pH=�Clg0.01=2��pHֵΪ8��NaOH��Һ��c(OH��)=10��6 mol��L�C1��pHֵΪ10��NaOH��Һ��c(OH��)=10��4 mol��L�C1���������Ϻ�Ļ����Һ��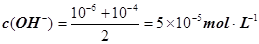 ��������Ũ��Ϊ
��������Ũ��Ϊ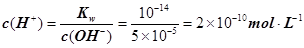 ����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4�� ����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)=
����3�� ��10�C14 mol��L�C1����10�C13 mol��L�C1����10�C12 mol��L�C1����10�C11 mol��L�C1������ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U��=1�U10�U100�U1000����ΪA����4�� ����c(OH�C)=10�C2 mol��L�C1��c(H+)=10�C11 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13������pH=3���c(H+)=10�C3 mol��L�C1����Kw= c(H+)��c(OH�C)=10�C13���c(OH�C)=10�C10 mol��L�C1�����Ը���Һ��c(H��)��c(OH��)��10�C3��10�C10��107��1���������е�c(H+)=10�C2 mol��L�C1������������Һ��c(OH�C)=10�C2 mol��L�C1������Һ��������ʱ�����ǡ����ȫ�кͣ���Һ�����ԣ�c(H+)= 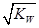 = 10�C6.5 mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)=5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C) =Kw/c(OH�C)=5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)=Kw/c(H+)=5.0��10�C12 mol��L�C1��
= 10�C6.5 mol��L�C1����pH=6.5����5��ˮ�����������Ũ�����ǵ�������������������Ũ�ȣ�����c(OH�C)=c(H+)=5.0��10�C7 mol��L�C1�����¶���Kw= c(H+)��c(OH�C)=5.0��10�C7��5.0��10�C7 =2.5��10�C13����c(H+)=5.0��10�C3 mol��L�C1����c(OH�C) =Kw/c(OH�C)=5.0��10�C11 mol��L�C1����c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)=Kw/c(H+)=5.0��10�C12 mol��L�C1��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ϊ0.10mol��L-1 NH4Cl��NH4HSO4��Һ��c(NH4+)ǰ��С�ں��� |
| B��25��ʱNH4Cl��Һ��KW����100��ʱNH4Cl��Һ��KW |
| C��25��ʱpH=11��NaOH��pH=11�İ�ˮ�ֱ�ϡ��100�����pHǰ��һ�����ں��� |
| D��25��ʱ��pH��4��������pH��10�İ�ˮ��Һ�������Ϻ�pH< 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮú����һ����Դ | B��ˮ���Ƕ�����Դ |
| C����Ȼ���Ƕ�����Դ | D�������Ƕ�����Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��c (CH3COO��)��c (Na+)��c (H+)��c (OH��) | B��c (CH3COO��)��c (Na +)��c (OH��)��c (H+) |
| C��c (CH3COO��)��c (H+)��c (Na+)��c (OH��) | D��c (Na+)��c(CH3COO��)��c (OH��)��c (H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����pH=l��CH3COOH��Һ��pH=13��NaOH��Һ�������϶��� |
| B����1mL0��1 mol��L��1CH3COOH��Һ��10 mL 1 mol��L��1��NaOH��Һ��϶��� |
| C����0��1mol��L��1��CH3COOH��Һ��0��1 mol��L��1��NaOH��Һ�������϶��� |
| D����0��1 mol��L��1��CH3COONa��Һ��0��1 mol��L��1��NaOH��Һ�������϶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2.4��10��7mol/L | B��0.1��10��7mol/L |
C�� mol/L mol/L | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ȼ�����ǽ�ˮ��Ϊ�͵�����ȼ�� |
| B�������Ǿ�����ֵ�ߡ�����Ⱦ���ŵ��ȼ�� |
| C���Ҵ��DZ���������������������ȼ�� |
| D��ʯ�ͺ�ú�ǹ�������ʹ�õĿ������Ļ�ʯȼ��] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c��NH4+��=c��SO42���� |
| B��c��NH4+����c��SO42���� |
| C��c��NH4+����c��SO42���� |
| D��c��NH4+����c��SO42������c��H+��=c��NH4���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com