



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
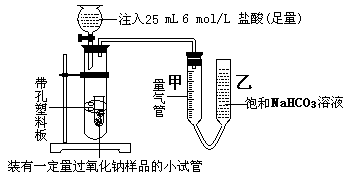
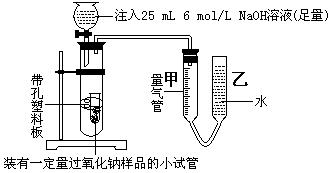
| | �����Լ�A | Һ���Լ�B |
| �� | | |
| �� | | |
| �� | | |
| �� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
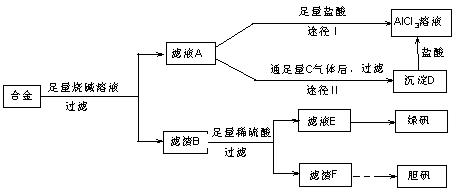
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
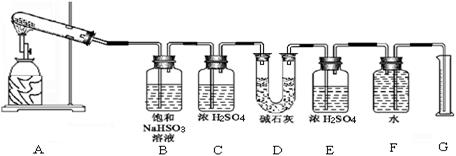
 �������ϴ����������� ��
�������ϴ����������� ��| ʵ��С�� | ��ȡCaSO4 ������(g) | װ��D���� ������(g) | ��ȡ���������װ�ò������������ (����ɱ�״������������) (mL) |
| һ | 4.08 | 2. 56 56 | 224 |
| �� | 5.44 | 2.56 | 448 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| �¶�/�� | 0 | 10 | 20 | 30 | 50 | 80 | 100 |
| �ܽ��(g/100gH20) | 74.4 | 81.9 | 91.8 | 106.8 | 315.1 | 525.8 | 535.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�| A��BaCl2 | B��NaOH | C��Na2SO4 | D��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
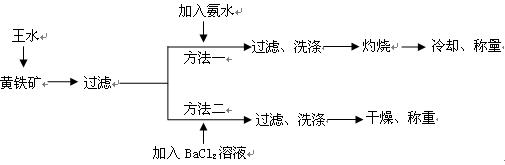
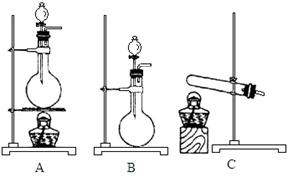
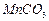 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������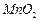 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ�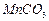 �IJ����������£�
�IJ����������£�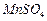 ��Һ��
��Һ��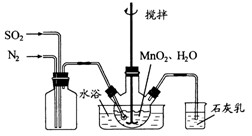
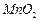 ��ˮ�����裬ͨ��
��ˮ�����裬ͨ��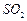 ��
��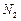 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��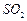 ��������ӦƬ�̣����ˣ���֪
��������ӦƬ�̣����ˣ���֪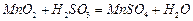 ����
����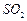 ������ת����ȫ����ͨ��
������ת����ȫ����ͨ��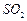 ��
��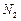 ����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��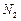 ���ɿ�������÷�ӦҺ��
���ɿ�������÷�ӦҺ��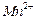 ��
��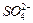 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��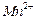 ��
��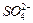 Ũ�ȱ仯�������Բ����ԭ���� ��
Ũ�ȱ仯�������Բ����ԭ���� ��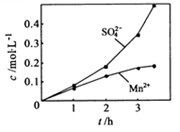
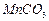 ���壺��֪
���壺��֪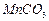 ������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻
������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻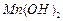 ��ʼ����ʱ
��ʼ����ʱ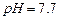 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�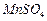 ��Һ�Ʊ��ߴ�
��Һ�Ʊ��ߴ�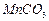 �IJ�������[ʵ���п�ѡ�õ��Լ���
�IJ�������[ʵ���п�ѡ�õ��Լ���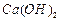 ��
��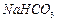 ��
��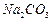 ��
��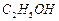 ]��
]���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������Գ�ȥ�������е�����̼�������� |
| B���ýྻ�IJ�����պȡ��Һ������ʪ���pH��ֽ�ϲⶨ��pH |
| C����2.0mLŨ�Ⱦ�Ϊ0.1mol��L-1��KCl��KI�����Һ�еμ�1~2��0.01mol��L-1 AgNO3��Һ���������ʻ�ɫ��˵��AgCl��Ksp��AgI��Ksp�� |
| D������25.00ml��ʽ�ζ�����ȡ20.00ml ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��ʢ��10��0.1mol/LAgNO3��Һ���Թ��еμ�0.1mol/LNaCl��Һ���������г������ɣ��������еμ�0.1mol/LNa2S��Һ | ֤��AgCl��ת��Ϊ�ܽ�ȸ�С��Ag2S |
| B | ��2mL�ױ��м���3��KMnO4������Һ������2 mL���м���3��KMnO4������Һ���� | ֤���뱽�������ļ��ױ����� |
| C | ��Na2SiO3��Һ��ͨ��CO2 | ֤��̼������Աȹ���ǿ |
| D | �������Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ������ | ��֤������ˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com