
���� ��1�����ݻ��ϼ۵����������ϼ۽��͵�Ԫ�ر���ԭ��
��2�������軯�ؼ��������ӣ�
��3����������Ϊ������Ⱦ������ŷţ��������Ʊ�������������Σ�
��4�����ݶ����������Ư���ԣ���Ư�ɻָ���
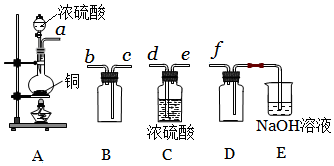
��5��ʵ�����Ʊ����ռ������SO2����װ�ÿ�֪��AΪ��Ӧװ�ã�CΪ����װ�ã�BΪ�ռ�װ�ã�DΪ��ֹ������EΪβ���������ݴ˷������
��� �⣺��1��8CuFeS2+21O2 $\frac{\underline{\;����\;}}{\;}$8Cu+4FeO+2Fe2O3+16SO2�У�CuԪ�صĻ��ϼ���+2�۽���Ϊ0��OԪ�صĻ��ϼ���0����Ϊ-2�ۣ����ϼ۽��͵�Ԫ�ر���ԭ����Cu��O����ԭ��
�ʴ�Ϊ��Cu��O��
��2����ϡH2SO4����������Ӧ��ȡͭ��ʣ��Ĺ��������ȡ����������Һ��������Һ�д���Fe3+�ķ�����ȡ������Һ���μ�KSCN��Һ����Һ��죻
�ʴ�Ϊ��ȡ������Һ���μ�KSCN��Һ����Һ��죻
��3����������Ϊ������Ⱦ����ܸ߿��ŷţ���Ũ�����Ӧ���������Ʊ�������������Σ�ֻ��bc���ϣ�
�ʴ�Ϊ��bc��
��4����֤��ͭ��ұ��ͭ�ķ�Ӧ�������к���SO2�ķ����ǽ�����ͨ��Ʒ����Һ�У����Ʒ����Һ��ɫ�����Ⱥ��ֱ�죬��֤����SO2��
�ʴ�Ϊ��������ͨ��Ʒ����Һ�У����Ʒ����Һ��ɫ�����Ⱥ��ֱ�죬��֤����SO2��
��5����װ��A����SO2����Ӧ�Ļ�ѧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ CuSO4+SO2��+2H2O��
�ڸ���װ��AΪ��Ӧװ�ã�CΪ����װ�ã�BΪ�ռ�װ�ã�DΪ��ֹ������EΪβ���������������������Ӹ������ӿڣ�˳��Ϊa��d��e��c��b��f��װ��E��NaOH��Һ�����������ն����SO2����ֹ��Ⱦ���������������ӷ���ʽΪ��SO2+2OH=SO32++H2O��
�ʴ�Ϊ��d��e��c��b�����ն����SO2����ֹ��Ⱦ������SO2+2OH=SO32++H2O��
���� ���⿼�������ʵ��Ʊ����漰����������Ʊ������ӵļ��顢װ�õ�ѡ���֪ʶ�㣬�����Ʊ�ԭ����ʵ��װ�á�ʵ�鼼��Ϊ���Ĺؼ�����Ŀ�ѶȲ���ע������������ʻ���֪ʶ��


 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ժ����û�������к���0.07 molHCl | |
| B�� | �����Ժ����û���������С��ԭ��������������ͬ�����£� | |
| C�� | ����������Һ�У�NaCl��NaClO�����ʵ�����Ϊ5��2 | |
| D�� | ����������Һ�У�������0.02 molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeO | B�� | Fe2O3 | C�� | CuO | D�� | Cu2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�� | ���� | |
| A | ���ȷ��������е�С���� | �����ۻ��ɹ�����С��ȼ��ʱ������Ϊ��ɫ��ȼ�պ����ɵ���ɫ�Ĺ��� |
| B | �ھƾ����ϼ������� | �����ۻ���ʧȥ�����ۻ��������������䣬������һ��Ĥ���� |
| C | �ڿ����о��õ���������NaOH��Һ�� | ���̲���������ɫ���ݣ�������ϸ���������� |
| D | þ����CO2��ȼ�� | ����ȼ�գ��ų��������ȣ�����ҫ�۰⣬���ɰ�ɫ����ͺ�ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
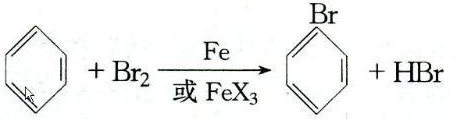 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
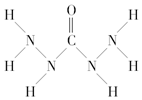
| A�� | A����Ԫ�طǽ�������ǿ����ֻ����Ԫ���Ը��� | |
| B�� | ��A�е�����Ԫ����ɵĻ�������������ӻ����� | |
| C�� | A�����д�������ۼ� | |
| D�� | A�����е�ԭ��û�йµ��ӶԶ���ԭ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3��NaHCO3�����������NaOH��Һ��Ӧ | |
| B�� | NaHCO3�����л��е�Na2CO3���ü��ȵķ�����ȥ | |
| C�� | Na2O2��Na2O���������Ӹ����Ⱦ�Ϊ1��2 | |
| D�� | �ֱ���Na2O2��Na2O��ˮ��Ӧ�����Һ�����������̪��Һ��������ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com