
��A��ȫȼ�����ɵĶ�����̼�����ʵ�����ˮ�����ʵ����٣�0.1molA��ȫȼ�պ��������ȫ������ʯ�����գ�ʹ��ʯ������26.6g��A��һ��ȡ��������4���칹�壬B1��B2�������е����֡���B2���к˴Ź�����������ֻ��һ�����շ塣�������ͼ��ʾ�ĸ��л���֮���ת����գ�
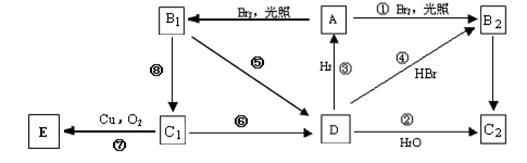
��1�������١��෴Ӧ�У�����ȡ����Ӧ����___ __�����ڻ�ԭ��Ӧ���� ������ţ���
��2������C2�����ƣ�
��3��д���ٵĻ�ѧ����ʽ��
��4��д���ݵĻ�ѧ����ʽ�� ����Ӧ����Ϊ
��5��д���ߵĻ�ѧ����ʽ��
��6������D��һ�ֺ����ü�ֵ���л�ԭ�ϡ����Ծۺϣ����ɸ߷������ʣ�д����Ӧ�Ļ�ѧ����ʽ ��D��ij�鷢���������Ӧ���ɷ���ʽΪC8H18������F��F��һ±����ֻ��4�֣���̼�����Գơ�
д��F�Ľṹ��ʽ ������Ϊ ��
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O(H+) |
 ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��







| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |

 ��R��R?������
��R��R?������







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
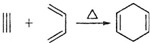 �����Ҫ�ϳ�
�����Ҫ�ϳ�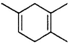 ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����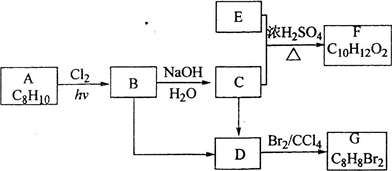
| NaOH |
| �� |
| NaOH |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ɽ��������߿���ѧ����һ�֣� ���ͣ�058
��ѧ����1978���Ƶ�һ����A��A�ɿ�������B��������ԭ�ӱ���C��һ�ۻ�ȡ�����ã�A��Br2��CCl4��Һ����ɫ��A����ԭ�ӱ�һ����ԭ��ȡ��ֻ��һ�����ʣ�һ������C��ȫȼ�����õ�H2O��CO2�����ʵ���֮��Ϊ1.25��C���칹�岻����3�֣���C�Ķ������Ϊ3�֣�һ������B��ȫȼ�����ɵ�CO2��H2O�����ʵ���֮��Ϊ2��B����Է�����������26��С��78��
�Ը���������Ϣ�ƶ�A��B��C�Ļ�ѧʽ����д��A�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��B�ķ���ʽΪ__________��
��2��д��C�Ľṹ��ʽ__________��
��3��A�ķ���ʽΪ__________��A�Ľṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com