
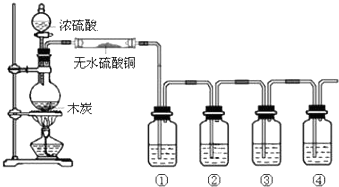
���� Ũ�����̼�ڼ��������·�Ӧ���ɶ�����̼�����������ˮ����֤���ɲ���ɶ�����̼�����������ˮ����Ҫ��������ˮ����ͭ����ˮ���������ɣ������âټ����������������Ʒ����Һ��ɫ֤������װ�����ø��������Һ��ȥ��������װ������Ʒ����Һ������������Ƿ��������װ�������ó���ʯ��ˮ�����֤��������̼�Ĵ��ڣ��ݴ˻ش�
��� �⣺Ũ�����̼�ڼ��������·�Ӧ���ɶ�����̼�����������ˮ����֤���ɲ���ɶ�����̼�����������ˮ����Ҫ��������ˮ����ͭ����ˮ���������ɣ������âټ����������������Ʒ����Һ��ɫ֤������װ�����ø��������Һ��ȥ��������װ������Ʒ����Һ������������Ƿ��������װ�������ó���ʯ��ˮ�����֤��������̼�Ĵ��ڣ�
��1��ͼ��4��ϴ��ƿװ�е��Լ������Ǣ�Ʒ����Һ���ڸ��������Һ����Ʒ����Һ���ܳ���ʯ��ˮ���ʴ�Ϊ��Ʒ����Һ�����������Һ��Ʒ����Һ������ʯ��ˮ��
��2�������������������Ʒ����Һ��ɫ֤�������������ò��ﺬSO2��ʵ������Ϊ����Ʒ����Һ��ɫ���ʴ�Ϊ������Ʒ����Һ��ɫ��
��3���������м���Ũ������Խ��裬Ũ���������ˮ�ԣ���ȥˮ��õ�̼���ʣ�ʵ������Ϊ���DZ�ڣ�����Ũ�������ˮ�ԣ��ʴ�Ϊ�����DZ�ڣ���ˮ�ԣ�
���� ���⿼����Ũ�������ʵķ����жϣ������ʵ����ƺ�ʵ����֤�������Լ�ѡ��Ӧ������жϣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH���� | B�� | Na2CO3���� | C�� | NaCl���� | D�� | CH3COONa���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�ǵ����Եģ������Ǵ���� | |
| B�� | CO2��ˮ��Һ�ܵ��磬����CO2Ϊ����� | |
| C�� | ��ʹ�ö����ЧӦ������FeCl3��Һ��Fe��OH��3���� | |
| D�� | 1 mol �κ����ʾ�����Լ6.02��1023��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ag+��K+��NO3-��Cl- | B�� | Mg2+��Na+��Cl-��SO42- | ||
| C�� | Ca2+��Mg2+��OH-��Cl- | D�� | H+��Na+��CO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
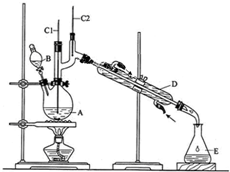
| �е�/�� | �ܶ�/g•cm-3 | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | �٢ۢݢܢ� | C�� | �٢ݢܢۢ� | D�� | �٢ڢݢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com