
CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
![]()
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
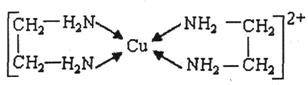
��ش��������⣺
��1����̬Cuԭ�ӵĺ�������Ų�ʽΪ ��H��N��O����Ԫ�صĵ縺���ɴ�С��˳���� ��
��2��SO2���ӵĿռ乹��Ϊ____________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ
��
 ��3���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��
��3���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��
��4���������γɵ��������к��еĻ�ѧ��������_______��(����ĸ)
a����λ�� b�����Լ� c�����Ӽ� d���Ǽ��Լ�
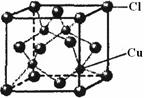
��5��CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_____��
 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�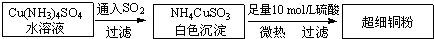
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ�
�ų�ϸͭ�۵�ij�Ʊ��������£�
��[Cu(NH3)4]SO4�������Ļ�ѧ���� �� �� ��
N��O��S����Ԫ�صĵ�һ�����ܴ�С˳��Ϊ�� �� �� ������Ԫ�ط��ţ�
��NH4CuSO3�еĽ��������ӵĺ�������Ų�ʽΪ�� ��
�� NH3������Nԭ�ӵ��ӻ���ʽΪ�� ��
����SO2��Ϊ�ȵ�����ķ����� ��дһ�֣���
���Ȼ���ͭ��CuCl����ij�Ʊ������ǣ���CuCl2��Һ��ͨ��һ����SO2���ȣ���Ӧһ��ʱ�������CuCl��ɫ������
��CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ_________��
��CuCl���۵��CuO���۵� ������ߡ��͡���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com