
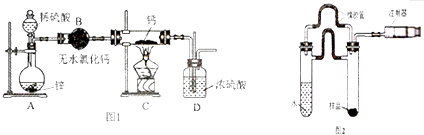
���� �⻯�Ƶ��Ʊ���п��ϡ���ᷴӦ��Zn+H2SO4=ZnSO4+H2���������к���ˮ������ͨ����ˮ�Ȼ��ƽ��и����װ��C�кƽ��з�ӦCa+H2$\frac{\underline{\;\;��\;\;}}{\;}$CaH2�������⻯�ƣ����⻯����ˮ������Ӧ�����������ƺ�����������D��ֹ�����е�ˮ��������C�У�
��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը��
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y�����ݷ���ʽCaH2+2H2O�TCa��OH��2+2H2����Ca+2H2O�TCa��OH��2+H2�����з��̼���x��y��ֵ���ٸ�����������������㣮
��� �⣺��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���װ��B�������ǣ���ȥ�����е�ˮ������װ��D�������ǣ���ֹ�����е�ˮ��������Cװ�ã�
�ʴ�Ϊ����ȥ�����е�ˮ��������ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը������ȷ�IJ���˳��Ϊ���ڢ٢ܢۣ�
�ʴ�Ϊ���ڢ٢ܢۣ�
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
�ʴ�Ϊ����ˮ����ͭ��
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
�ʴ�Ϊ��Na2CO3��ϴ�ӡ���ɣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y����
CaH2+2H2O�TCa��OH��2+2H2��
42 4
X Y
����42��4=x��y��������y=$\frac{2x}{21}$
Ca+2H2O�TCa��OH��2+H2��
40 2
46mg-x 4.2mg-y
����40��2=��46mg-x������4.2mg-y������y=$\frac{2x}{21}$���룬���x=42mg��������Ʒ���⻯�ƵĴ���Ϊ��$\frac{42mg}{46mg}$��100%=91.30%��
�ʴ�Ϊ��91.3%��
���� �������⻯���Ʊ�Ϊ���壬����ʵ���������������������е���Ϣ�����á���ʵ��װ�õ�������������ʷ����ᴿ����ѧ���㡢ʵ�鷽����Ƶȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu2+��NO3-��Cl-��Na+ | B�� | NH4+��Mg2+��NO3-��SO42- | ||
| C�� | K+��Ca2+��HCO3-��Cl-�� | D�� | Cl-��SO42-��K+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʮһ�� | B�� | ����ʮ���� | C�� | ����ʮ���� | D�� | ����ʮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ���� | T/K | ��Ӧʱ��/h | ��Ӧ������ʵ������n[CO��NH2��2]��n[MgCl2.6H2O] | ʵ��Ŀ�� |
| �� | 378 | 3 | 3��1 | ��I��ʵ��ٺ͢�̽����Ӧ������ʵ�����ȶԲ��ʵ�Ӱ�� ��II��ʵ��ں͢�̽���¶ȶԲ��ʵ�Ӱ�� ��III��ʵ��ں͢�̽����Ӧʱ��Բ��ʵ�Ӱ�� |
| �� | 378 | 4 | 4��1 | |
| �� | 378 | 3 | ||
| �� | 398 | 4 | 4��1 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| �⻯��ķе㣨�棩 | -60.7 | -33.4 | -111.5 | 100 | -87.7 | 19.54 | -84.9 | -161.5 |
| ����ϼ� | +6 | +5 | +4 | +5 | +7 | +4 | ||
��ͻ��ϼ� | -2 | -3 | -4 | -2 | -3 | -1 | -1 | -4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ά��������ϳ������أ�ͻ�����������л���Ľ��� | |
| B�� | ����ʽΪC4H8���л�����ܴ���4��C-C���� | |
| C�� | ��CH3��3CCH2CH��C2H5��CH3 ������Ϊ2��2-����-4-�һ����飬�˴Ź���������6��� | |
| D�� | ������������Ȼ��ABS��֬�����ɸ߷��ӻ�������ɵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
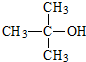
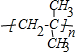
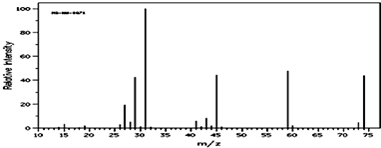
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com