


 æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢¢Ł¢Ś¢Ū¢Ü | B”¢¢Ł¢Ū¢Ü |
| C”¢¢Ś¢Ū¢Ü | D”¢¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| AӢPCl3 |
| BӢSO3 |
| CӢBF3 |
| DӢCS2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
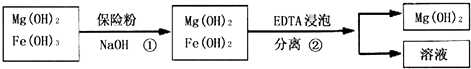
| ¾«ÖĘ×čČ¼¼ĮµÄĢõ¼ž | ×čČ¼¼ĮĢśŗ¬Įæ | |||
| ŠņŗÅ | Ģį“æĢåĻµĪĀ¶Č/”ę | ¼ÓČėEDTAÖŹĮæ/g | ¼ÓČė±£ĻÕ·ŪÖŹĮæ/g | W£ØFe£©/£Ø10-4g£© |
| 1 | 40 | 0.05 | 0.05 | 7.63 |
| 2 | 40 | 0.05 | 0.10 | 6.83 |
| 3 | 60 | 0.05 | 0.10 | 6.83 |
| 4 | 60 | 0.10 | 0.10 | 6.51 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÓĆ0.1320mol/LµÄHClČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ£¬ŹµŃ鏿¾ŻČēĻĀ±ķĖłŹ¾£¬
ÓĆ0.1320mol/LµÄHClČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄNaOHČÜŅŗ£¬ŹµŃ鏿¾ŻČēĻĀ±ķĖłŹ¾£¬| ŹµŃ鱹ŗÅ | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | HClČÜŅŗµÄĢå»ż/mL |
| 1 | 25.00 | 24.41 |
| 2 | 25.00 | 24.39 |
| 3 | 25.00 | 22.60 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| µĪ¶ØŠņŗÅ | “ż²āŅŗĢå»ż | ĖłĻūŗÄŃĪĖį±ź×¼µÄĢå»ż£ØmL£© | |
| µĪ¶ØĒ° | µĪ¶Øŗó | ||
| 1 | 25.00 | 0.50 | 20.60 |
| 2 | 25.00 | 6.00 | 26.00 |
| 3 | 25.00 | 1.10 | 21.00 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com