
| ĪļĄķŠŌÖŹ | ŃÕÉ« | דĢ¬ | ČܽāŠŌ | ČŪµć | ·Šµć | ĆÜ¶Č |
| äåŅŅĶé | ĪŽÉ« | ŅŗĢå | ÄŃČÜÓŚĖ® | 38.4”ę | 119”ę | 1.46g/cm3 |
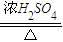 C2H5Br+H2O
C2H5Br+H2O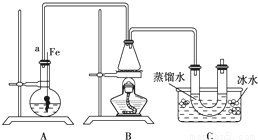
 +Br2
+Br2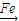
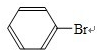 +HBr£¬
+HBr£¬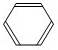 +Br2
+Br2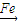
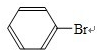 +HBr£»
+HBr£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
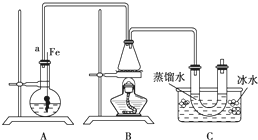 Ä³Ń§Éś²éŌÄ׏ĮĻµĆÖŖäåŅŅĶéµÄĪļĄķŠŌÖŹÓėÖĘČ”·½·ØČēĻĀ±ķ£ŗ
Ä³Ń§Éś²éŌÄ׏ĮĻµĆÖŖäåŅŅĶéµÄĪļĄķŠŌÖŹÓėÖĘČ”·½·ØČēĻĀ±ķ£ŗ| ĪļĄķŠŌÖŹ | ŃÕÉ« | דĢ¬ | ČܽāŠŌ | ČŪµć | ·Šµć | ĆÜ¶Č |
| äåŅŅĶé | ĪŽÉ« | ŅŗĢå | ÄŃČÜÓŚĖ® | 38.4”ę | 119”ę | 1.46g/cm3 |
| ||
| ”÷ |
 +Br2
+Br2| Fe |
 +HBr
+HBr +Br2
+Br2| Fe |
 +HBr
+HBr²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½Ī÷Ź”“óĶ¬ŹµŃé֊ѧø߶ž£ØĻĀ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
| ĪļĄķŠŌÖŹ | ŃÕÉ« | דĢ¬ | ČܽāŠŌ | ČŪµć | ·Šµć | ĆÜ¶Č |
| äåŅŅĶé | ĪŽÉ« | ŅŗĢå | ÄŃČÜÓŚĖ® | 38.4”ę | 119”ę | 1.46g/cm3 |
 C2H5Br+H2O
C2H5Br+H2O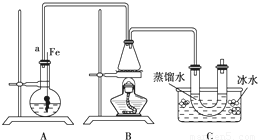
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ½Ī÷Ź”ÉĻø߶žÖŠø߶žĻĀѧʌµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©Ä³Ń§Éś²éŌÄ׏ĮĻµĆÖŖäåŅŅĶéµÄĪļĄķŠŌÖŹÓėÖĘČ”·½·ØČēĻĀ±ķ£ŗ
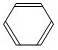
ĮŖĻėµ½ĖłŃ§äå±½µÄÖĘČ”£¬ĖūÉčĮĖÖĘČ”äå±½ŗĶäåŅŅĶéµÄ×°ÖĆI£¬Ö÷ŅŖŹµŃé²½ÖčČēĻĀ£ŗ
¢Ł¼ģ²éĘųĆÜŠŌŗó£¬ĻņÉÕĘæÖŠ¼ÓČėŅ»¶ØĮæµÄ±½ŗĶŅŗä唣
¢ŚĻņ׶ŠĪĘæÖŠ¼ÓČėŅŅ“¼ŗĶÅØĮņĖįµÄ»ģŗĻŅŗÖĮĒ”ŗĆÓŚ½ųĘųµ¼¹ÜæŚ”£
¢Ū½«A×°ÖĆÖŠµÄ“æĢśĖæĻņĻĀ²åČė»ģŗĻŅŗÖŠ”£
¢ÜµćČ¼B×°ÖĆÖŠ¾Ę¾«µĘ£¬ÓĆŠ”»š»ŗ»ŗ¶Ō׶ŠĪĘæ¼ÓČČ10min.ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ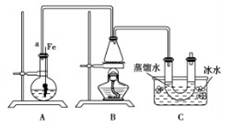
£Ø1£©AÖŠ·¢Éś·“Ó¦µÄ·½³ĢŹ½
£Ø2£©×°ÖĆ³¤µ¼¹ÜaµÄ×÷ÓĆŹĒ
£Ø3£©C×°ÖĆÖŠµÄUŠĪ¹ÜÄŚÓĆÕōĮóĖ®·ā×”¹Üµ×µÄ×÷ÓĆŹĒ
£Ø4£©·“Ó¦Ķź±Ļŗó£¬UŠĪ¹ÜÄŚµÄĻÖĻóŹĒ ·ÖĄė³öäåŅŅĶ鏱ĖłÓƵÄ×īÖ÷ŅŖµÄŅĒĘ÷Ćū³ĘŹĒ £ØÖ»ĢīŅ»ÖÖ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com