
��10�֣������������С��
��1�������г��˼������ʣ���ѡ��������ʵ�������ڿո��ϡ�
ͬλ�� ��ͬϵ�� ��ͬ���칹�� ��
�� ���ʯ��ʯī ��CH3CH2CH2CH3��(CH3)2CHCH3
��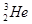 ��
��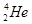 ��CH3CH3
��CH3CH2CH2CH3
��CH3CH3
��CH3CH2CH2CH3
 ��
��CH2��CHCH3��CH2��CH2��
��
��CH2��CHCH3��CH2��CH2��
��D��T������ ��������������������������������
��2���й��ľ��Ļ�ԨԴ�������Ŵ����DZ㶮��������ʳ����ʵ���������ƣ���д����������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��___________________��
���Ҵ��������Ļ�ѧ����ʽ��______________________________��
����10�֣�ÿ��2��)��1�� �ۢ� �ܢ� �ڢ�
��2����C6H12O6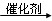 2CH3CH2OH��2CO2��
2CH3CH2OH��2CO2��
��2C2H5OH+O2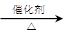 2CH3CHO+2H2O
2CH3CHO+2H2O
����������1����������ͬ����������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ���Ϊͬλ�أ����Դ��Ǣۢߣ��ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ���л���ģ�����Ϊͬϵ���˴��Ǣܢޣ�����ʽ��ͬ�ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬���Դ�ѡ�ڢࡣ
��2���������ڴ����������·ֽ⼴�����Ҵ���ͬʱ������CO2���Ҵ������ǻ�������������������ȩ��


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������һ�и�һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
��10�֣������������С��
��1�������г��˼������ʣ���ѡ��������ʵ�������ڿո��ϡ�
ͬλ�� ��ͬϵ�� ��ͬ���칹�� ��
�� ���ʯ��ʯī ��CH3CH2CH2CH3��(CH3)2CHCH3
�� ��
�� ��CH3CH3��CH3CH2CH2CH3
��CH3CH3��CH3CH2CH2CH3 �� ��CH2��CHCH3��CH2��CH2��
�� ��CH2��CHCH3��CH2��CH2��
��D��T������ ��������������������������������
��2���й��ľ��Ļ�ԨԴ�������Ŵ����DZ㶮��������ʳ����ʵ���������ƣ���д����������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��___________________��
���Ҵ��������Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������
��1�������г��˼������ʣ���ѡ��������ʵ�������ڿո��ϡ�
ͬλ�� ��ͬϵ�� ��ͬ���칹�� ��
�� ���ʯ��ʯī ��CH3CH2CH2CH3��(CH3)2CHCH3
��![]() ��
��![]() ��CH3CH3 ��CH3CH2CH2CH3
��CH3CH3 ��CH3CH2CH2CH3
 �� ��CH2��CHCH3��CH2��CH2��
�� ��CH2��CHCH3��CH2��CH2��
��D��T������ ��������������������������������
��2���й��ľ��Ļ�ԨԴ�������Ŵ����DZ㶮��������ʳ����ʵ���������ƣ���д����������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��___________________��
���Ҵ��������Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com