
����Ŀ�����Ϳ�����ҩ���ƥ�أ��ɱ���θ���Ĥ���ܸ������������ӵ�Σ������ϳ�·�����£�
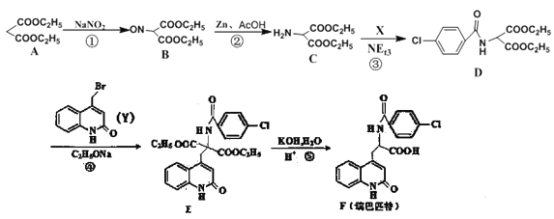
(1)A�Ļ�ѧ����Ϊ______________��C�ĺ˴Ź���������_________________���
(2)A��������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ______________
(3)������F�к��������ŵ�����Ϊ__________________��������F�ķ���ʽΪ_____________
(4)��Ӧ��~���У�����ȡ����Ӧ����__________________(�����)
(5)C��D��ת���У����ɵ���һ�ֲ���ΪHCl����C��D��Ӧ�Ļ�ѧ����ʽΪ__________
(6)��֪Y�е���ԭ�ӱ�--OHȡ���õ�Z��д��ͬʱ��������������Z��һ��ͬ���칹��Ľṹ��ʽ��___________________
I�������к���һ��������һ����Ԫ�����Ҷ���̼ԭ�ӻ�
II��������������ȡ�������Ҵ��ڶ�λ
III.����NaHCO3��Һ������Ӧ
(7)��֪CH3CH2OH![]() CH3CH2Br����A��HOCH2CH2CH2OHΪԭ���Ʊ�
CH3CH2Br����A��HOCH2CH2CH2OHΪԭ���Ʊ�![]() �ĺϳ�·������ͼ���£�HOCH2CH2CH2OH
�ĺϳ�·������ͼ���£�HOCH2CH2CH2OH![]() ����X
����X![]() ����Y
����Y![]()
![]() ��������XΪ__________������YΪ____________
��������XΪ__________������YΪ____________
���𰸡������������ 4 C2H5OOCCH2COOC2H5��2NaOH ![]() NaOOCCH2COONa��2C2H5OH �ļ����Ȼ� C19H15O4N2Cl �٢�
NaOOCCH2COONa��2C2H5OH �ļ����Ȼ� C19H15O4N2Cl �٢� 
 BrCH2CH2CH2Br
BrCH2CH2CH2Br 
��������
��1������ϵͳ���������л��� �Ļ�ѧ����Ϊ�������������C��
�Ļ�ѧ����Ϊ�������������C�� ������4����ԭ�ӣ��˴Ź���������4��壬
������4����ԭ�ӣ��˴Ź���������4��壬
�ʴ�Ϊ���������������4��
��2��������NaOH��Һ�з�������ˮ�⣬�����������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ![]() +2NaOH
+2NaOH![]() NaOOCCH2COONa+2C2H5OH���ʴ�Ϊ��
NaOOCCH2COONa+2C2H5OH���ʴ�Ϊ�� ![]() +2NaOH
+2NaOH![]() NaOOCCH2COONa+2C2H5OH��
NaOOCCH2COONa+2C2H5OH��
��3��������F�� ���й����ŵ�����Ϊ�ļ����Ȼ�����ԭ�ӡ�̼̼˫�������к��������ŵ�����Ϊ�ļ����Ȼ���������F�ķ���ʽΪC19H15O4N2Cl���ʴ�Ϊ���ļ����Ȼ���C19H15O4N2Cl��
���й����ŵ�����Ϊ�ļ����Ȼ�����ԭ�ӡ�̼̼˫�������к��������ŵ�����Ϊ�ļ����Ȼ���������F�ķ���ʽΪC19H15O4N2Cl���ʴ�Ϊ���ļ����Ȼ���C19H15O4N2Cl��
��4����Ӧ���� ����
���� �ķ�Ӧ����Ϊȡ����Ӧ����Ӧ����
�ķ�Ӧ����Ϊȡ����Ӧ����Ӧ���� ����ȥ����ԭ����
����ȥ����ԭ����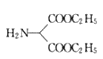 ���䷴Ӧ����Ϊ��ԭ��Ӧ����Ӧ����
���䷴Ӧ����Ϊ��ԭ��Ӧ����Ӧ���� �İ����е�1����ԭ�ӱ�
�İ����е�1����ԭ�ӱ�![]() ȡ������ȡ����Ӧ�����Է�Ӧ��~���У�����ȡ����Ӧ���Ǣ٢ۣ��ʴ�Ϊ���٢ۡ�
ȡ������ȡ����Ӧ�����Է�Ӧ��~���У�����ȡ����Ӧ���Ǣ٢ۣ��ʴ�Ϊ���٢ۡ�
��5���Ա�C��D�Ľṹ��֪��C��X����ȡ����Ӧ����D��HCl�����ݲ����̼�ܽṹ�����ԭ���غ㣬��֪C��D��Ӧ�Ļ�ѧ����ʽΪ�� +
+![]()
![]()
 +HCl��
+HCl��
�ʴ�Ϊ�� +
+![]()
![]()
 +HCl��
+HCl��
��6��Y�Ľṹ��ʽΪ��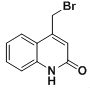 ��Y�е���ԭ�ӱ�-OHȡ���õ�Z����Z�Ľṹ��ʽΪ��
��Y�е���ԭ�ӱ�-OHȡ���õ�Z����Z�Ľṹ��ʽΪ��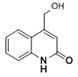 ��Z��ͬ���칹��I�������к���һ��������һ����Ԫ�����Ҷ���̼ԭ�ӻ���˵��Nԭ�Ӳ��ڻ��ϣ�III.����NaHCO3��Һ������Ӧ��˵�������к����Ȼ���II��������������ȡ������Ӧ���ǰ������Ȼ����Ҵ��ڶ�λ��ͬʱ��������������Z��һ��ͬ���칹��Ľṹ��ʽΪ
��Z��ͬ���칹��I�������к���һ��������һ����Ԫ�����Ҷ���̼ԭ�ӻ���˵��Nԭ�Ӳ��ڻ��ϣ�III.����NaHCO3��Һ������Ӧ��˵�������к����Ȼ���II��������������ȡ������Ӧ���ǰ������Ȼ����Ҵ��ڶ�λ��ͬʱ��������������Z��һ��ͬ���칹��Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(7)��֪CH3CH2OH![]() CH3CH2Br����A��HOCH2CH2CH2OHΪԭ���Ʊ�
CH3CH2Br����A��HOCH2CH2CH2OHΪԭ���Ʊ�![]() �ĺϳ�·������ͼ���£�
�ĺϳ�·������ͼ���£� ��������XΪBrCH2CH2CH2Br������YΪ��
��������XΪBrCH2CH2CH2Br������YΪ�� ���ʴ�Ϊ��BrCH2CH2CH2Br��
���ʴ�Ϊ��BrCH2CH2CH2Br�� ��
��


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��������ɱ���ܱ������з������·�Ӧ��A(g)+B(g)![]() 2C(g) ��H<0��t1ʱ�̴ﵽƽ�����t2ʱ�̸ı�ijһ�������䷴Ӧ������ͼ������˵����ȷ����
2C(g) ��H<0��t1ʱ�̴ﵽƽ�����t2ʱ�̸ı�ijһ�������䷴Ӧ������ͼ������˵����ȷ����

A. 0��t2ʱ��v������>v���棩
B. t2ʱ�̸ı�����������ǼӴ���
C. �����������̴ﵽ��Ӧ��ʱ��A�����������=��
D. �����������̴ﵽ��Ӧ��ʱ��ƽ�ⳣ��I<��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ҫ�Ļ���ԭ�ϣ���;�dz��㷺��
ʵ��һ��SO2��������ϸ�����������з�����Ч��ijʵ��С��������ͼ��ʾװ�òⶨijƷ�����Ѿ���(���Ѿ��к����Ҵ����л����)��SO2������
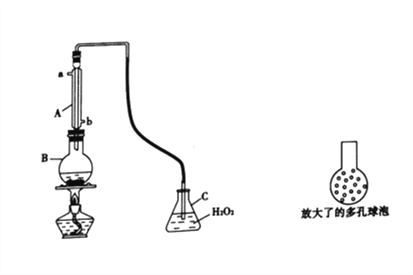
��1������A��������________��ʹ�ø�װ����ҪĿ����____________________��
��2��B�м��� 300.00 mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ��C�л�ѧ����ʽΪ_________________________________________��
��3��������Cװ���еĵ��ܶ��˸ijɾ��ж������(��ͼ15��ʾ)�������ʵ���ȷ�ȣ�������_______________________________________��
��4����ȥC�е�H2O Ȼ����0.099mol��L-1NaOH����Һ�ζ���
���ü�ʽ�ζ�����ȡ0.09mol��L-1NaOH����Һǰ��һ��������___________________________��
���ø÷����ⶨ���Ѿ���SO2�ĺ���ƫ�ߣ���Ҫԭ����__________________________________���������е�װ�ã�����Ľ��Ĵ�ʩ��_______________________________________________��
��5������C�е���Һ���кܶ�ʵ�鷽���ⶨ���Ѿ���SO2�ĺ���������0.1mol��L-1BaCl2��Һ��ʵ�����IJ��ޣ�����ʵ�鲽�裺________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ��
(1)1molN2(g)������O2(g)��Ӧ����NO2(g)������68 kJ������_______________________��
(2)1molCu(s)������O2(g)��Ӧ����CuO(s)���ų�157 kJ������________________________��
(3)���Ƿ���ʱ����(N2H4)��ȼ�ϣ�1mol N2H4(l)��O2(g)��ȼ�գ�����N2(g)��H2O(l)���ų�622 kJ����___��
(4)���͵���Ҫ�ɷ�������(C8H18)��1molC8H18(l)��O2(g)��ȼ�գ�����CO2(g)��H2O(l)���ų�5 518 kJ������______________________________��
(5)1molC(s)������H2O(g)��Ӧ����CO(g)��H2(g)������131.5kJ������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ƿ�ռ��������������������壬Ȼ���䵹����ˮ���С��ֱ���ͨ������O2��Cl2����ͼ��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�ࡣ(����ƿ��Һ�岻��ɢ)

(1)д��װ��E�з�Ӧ�����ӷ���ʽ��__________________________________________
(2)�����ʵ�������£�����Ħ�����ΪaL��mol��1����װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ____________��������Ӧ�ķ���ʽ____________________________��
(3)ͨ������ǰ��Fװ�õ�ˮ����μӼ�����ɫʯ����Һ���۲쵽��������_____________��ͨ���������ܹ۲쵽��ʵ��������_________________________________________��д����Ӧ���ܻ�ѧ����ʽ��_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʾ������Ԫ���У�W��X��Y��ZΪ������Ԫ�أ�������Ԫ�ص�ԭ������������֮��Ϊ22������˵����ȷ����

A. X��Y��Z����Ԫ����ͼ��⻯��ķе���������
B. ��X��Y��������Ԫ���γɵĻ�������ֻ���й��ۼ�
C. ��X��Y����Ԫ���γɵĻ����ﶼ������������
D. TԪ�صĵ��ʾ��а뵼������ԣ�T��ZԪ�ؿ��γɻ�����TZ4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£�����������M��Nͨ���ݻ�ΪVL���ܱ������н��з�Ӧ��M��N�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ������˵����ȷ����( )

A. 0��t2����M��ʾ��ƽ����Ӧ������2/t2��mol��L��1��min��1��
B. t1��t2�������ڵ�ѹǿ��С
C. �÷�Ӧ�ķ���ʽΪN![]() 2M
2M
D. t2��t3ʱ�̵Ļ�������ƽ����Է����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������ºϳ���ϩ��6H2(g)��2CO2(g)![]() CH2===CH2(g)��4H2O(g)����֪�¶ȶ�CO2��ƽ��ת���ʺʹ�����Ч�ʵ�Ӱ����ͼ������˵������ȷ����( )
CH2===CH2(g)��4H2O(g)����֪�¶ȶ�CO2��ƽ��ת���ʺʹ�����Ч�ʵ�Ӱ����ͼ������˵������ȷ����( )
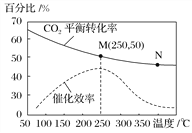
A. �÷�Ӧ���淴ӦΪ���ȷ�Ӧ
B. ƽ�ⳣ����KM>KN
C. ������ϩ�����ʣ�v(N)һ������v(M)
D. ���¶ȸ���250 �棬�����¶ȣ������Ĵ�Ч�ʽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ȼ�ѧ��Ӧ����������ȷ����(����)
A. ��֪NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.3 kJ��mol��1����74.0 g Ca(OH)2��ϡ��Һ��ϡ������ȫ�кͣ��ų�57.3 kJ������
B. ��֪N2(g)+3H2(g) ![]() 2NH3(g) ��H=-92.4kJ��mol-1����������N2(g)��H2(g)�����ܱյ������г�ַ�Ӧ����1mol NH3(g)���ų�46.2kJ������
2NH3(g) ��H=-92.4kJ��mol-1����������N2(g)��H2(g)�����ܱյ������г�ַ�Ӧ����1mol NH3(g)���ų�46.2kJ������
C. 2gH2��ȫȼ������Һ̬ˮ�ų�285.8 kJ���������ʾ����ȼ�յ��Ȼ�ѧ����ʽΪ:2H2(g)��O2(g)===2H2O(l)����H����571.6 kJ
D. ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH(g)��1/2O2(g)==CO2(g)��2H2(g)����H����192.9 kJ��mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ��mol��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com