


���� ��1��a��PM2.5��ָ������ֱ��С�ڻ����2.5��m�Ŀ����������Ҫ��Դ���ճ����硢��ҵ����������β���ŷŵȹ����о���ȼ�ն��ŷŵIJ����
b��PM2.5��������������ԣ������������ж����ʣ�
c��������ֱ����10-7m��10-9m֮�䣬PM2.5���ӵĴ�С�����ϣ�
d�����ٻ�����β���ŷţ��������̳����ܽ��Ϳ�����PM2.5��
��2�������������������ȡSO2���Լ�Ϊ��̬��Һ̬����Ӧ����������ȣ���ͨ������������������������Ʒ�Ӧ���ʣ�
��3��A��SO2����Ư���ԣ���ʹƷ����ֽ��ɫ��SO2���л�ԭ�ԣ������ǿ�����Ե�KMnO4����������ԭ��Ӧ��
B��SO2ֻ��ʹ���ָʾ����ɫ������Ư��ָʾ����
C������������+��ԭ������������+��ԭ��������ԣ�����������ԭ��������
D��SO2Ϊ�������壬����NaOH��Һ��Ӧ�����κ�ˮ��
��4����SO2��O2��Ӧ����1mol SO3����ʱ���ų�a kJ������д�Ȼ�ѧ����ʽ��
�ڸ��ݸ�˹���ɢ�2SO2��g��+O2��g���T2SO3��g����H=-2a kJ•mol-1��2NO��g��+O2��g���T2NO2��g����H=-b kJ•mol-1 ��a��b��0��$\frac{��-��}{2}$���ã�
��1������������˫��ˮ��Ӧ�������
��2�����ϲ���Һ�м����μ�Ba��OH��2��Һ���ǣ�
��װ�â��ж�������������������ܺ�ǿ����������֮�䷢����Ӧ��SO2+OH-=HSO3-��NO����������֮�䲻�ᷴӦ��
װ�â���NO�����������£�NO��Ce4+֮��ᷢ��������ԭ��Ӧ��NO+H2O+Ce4+=Ce3++NO2-+2H+��NO+2H2O+3Ce4+=3Ce3++NO3-+4H+��
װ�â��У��ڵ��۵�����2Ce3+-2e-=2Ce4+�������缫��ӦʽΪ��2HSO3-+2H++2e-=S2O42-+2H2O��
װ�â���ͨ�백����������2NO2-+O2+2H++2NH3=2NH4++2NO3-��
��1�������Ի����£�NO��Ce4+֮��ᷢ��������ԭ��Ӧ��
��2���ڵ����У������Ϸ�ʧȥ���ӵ�������Ӧ�������Ϸ����õ��ӵĻ�ԭ��Ӧ��
��3��NO2-��Ũ��Ϊ0.4mol/L��Ҫʹ1m3����Һ�е�NO2-��ȫת��ΪNH4NO3�������ı���������������V����ϵ����غ���м��㣮
��� �⣺��1��a��PM2.5��ָ������ֱ��С�ڻ����2.5��m�Ŀ����������Ҫ��Դ���ճ����硢��ҵ����������β���ŷŵȹ����о���ȼ�ն��ŷŵIJ������a��ȷ��
b��PM2.5��������������ԣ������������ж����ʣ���b����
c��������ֱ����10-7m��10-9m֮�䣬PM2.5���ӵĴ�С�����ϣ���c����
d�����ٻ�����β���ŷţ��������̳����ܽ��Ϳ�����PM2.5����d��ȷ��
��ѡ��bc��
��2����������������Ʒ�Ӧ��ȡ��������ϣ���ܿ��Ʒ�Ӧ�ٶȣ����ڷ�Ӧ����Ҫ���ȣ��ų�װ��c����������������ϸС������������ˮ������ѡ��װ��bd���ʿ�ѡ�õķ���װ����a��
�ʴ�Ϊ��a��
��3��A��Ʒ����ֽ��ɫ�����ֳ�SO2��Ư���ԣ�մ��KMnO4��Һ����ֽ��ɫ�����ֳ�SO2�Ļ�ԭ�ԣ���A���� B��SO2ֻ��ʹ���ָʾ����ɫ������ʪ�����ɫʯ����ֻֽ��죬��B����
C��ʪ�����KI��ֽ������˵��SO2�ܽ�KI����ΪI2����SO2��������ǿ��I2����C����
D��SO2Ϊ�������壬�ж�������NaOH��Һ��Ӧ�����κ�ˮ������NaOH��Һ�����ڳ�ȥʵ���ж����SO2����D��ȷ��
��ѡD��
��4����SO2��O2��Ӧ����1mol SO3����ʱ���ų�a kJ���������Ȼ�ѧ����ʽΪ��2SO2��g��+O2��g���T2SO3��g����H=-2a kJ•mol-1���ʴ�Ϊ��2SO2��g��+O2��g���T2SO3��g����H=-2a kJ•mol-1��
�ڸ��ݸ�˹���ɢ�2SO2��g��+O2��g���T2SO3��g����H=-2a kJ•mol-1��2NO��g��+O2��g���T2NO2��g����H=-b kJ•mol-1 ��a��b��0��$\frac{��-��}{2}$����NO2��g��+SO2��g���TSO3��g��+NO��g�� �ġ�H=$\frac{-2a+b}{2}$=-��a-$\frac{b}{2}$��kJ•mol-1���ʴ�Ϊ��-��a-$\frac{b}{2}$����
��1������������˫��ˮ��Ӧ�������ᣬ���ӷ���ʽΪSO2+H2O2=SO42-+2H+���ʴ�Ϊ��SO2+H2O2=SO42-+2H+��
��2�����ϲ���Һ�м����μ�Ba��OH��2��Һ���ǣ��ʴ�Ϊ�����÷ֲ�����ϲ���Һ�м����μ�Ba��OH��2��Һ���������������˵��Ba��OH��2�����������㣻
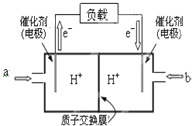
��װ�â��ж�������������������ܺ�ǿ����������֮�䷢����Ӧ��SO2+OH-=HSO3-��NO����������֮�䲻�ᷴӦ��װ�â���NO�����������£�NO��Ce4+֮��ᷢ��������ԭ��Ӧ��NO+H2O+Ce4+=Ce3++NO2-+2H+��NO+2H2O+3Ce4+=3Ce3++NO3-+4H+��װ�â��У��ڵ��۵�����2Ce3+-2e-=2Ce4+�������缫��ӦʽΪ��2HSO3-+2H++2e-=S2O42-+2H2O��װ�â���ͨ�백����������2NO2-+O2++2H++2NH3=2NH4++2NO3-��
��1��װ�â���NO������������NO��Ce4+֮��ᷢ��������ԭ��Ӧ��NO+H2O+Ce4+=Ce3++NO2-+2H+��NO+2H2O+3Ce4+=3Ce3++NO3-+4H+��
�ʴ�Ϊ��NO+H2O+Ce4+=Ce3++NO2-+2H+��
��2�����ص����������õ��ӵĻ�ԭ��Ӧ�����Ҳ�缫��ӦʽΪ2HSO3-+2H++2e-=S2O42-+2H2O�������缫��ӦΪ��Ce3+-e-�TCe4+����ͼ��AΪ��Դ��������
�ʴ�Ϊ������2HSO3-+2H++2e-=S2O42-+2H2O��
��3������Ce4+Ϊ������Ӧ�������������ϣ��������ʱ���ɵ�Ce4+�ڵ��۵����������ӵ�Դ��������Ӧ����HSO3-����ԭ��S2O42-���õ����ӣ��缫��ӦʽΪ��2HSO3-+2H++2e-=S2O42-+2H2O��
�ʴ�Ϊ������2HSO3-+2H++2e-=S2O42-+2H2O��
��4��NO2-��Ũ��Ϊ0.4mol/L��Ҫʹ1m3����Һ�е�NO2-��ȫת��ΪNH4NO3����ʧȥ������Ϊ��1000����5-3����0.4mol�������ı���������������V�����ݵ����غ㣺$\frac{VL}{22.4mol/L}$��4=1000����5-3����0.4mol�����V=4480L��
�ʴ�Ϊ��4480��
���� ���⿼����PM2.5��������γɡ���ҵ�����л�ѧԭ������SO2��NO�Ĺ��ա��Ȼ�ѧ����ʽ����д����˹���ɵ�Ӧ�á��Լ���ѧ����֪ʶ���Ѷ��еȣ�ץ�û����ǹؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����һС��ͭƬ | B�� | ���õ���� 98%������ | ||
| C�� | �õ������۴�����Ƭ | D�� | ���õ����3mol/L���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
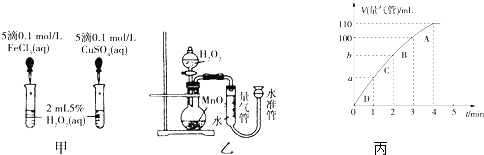
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
| ���� | Fe 2+ | Cu2+ | Mg2+ |
| pH | 7.6 | 5.2 | 10.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㣮
Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㣮| ��ѧ�� | H-H | N-H | N��N |
| ����/kJ•mol-1 | 436 | a | 945 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��1mL0.2mol/LNaOH��Һ�е���2��0.1mol/LMgCl2��Һ��������ɫ�������ٵμ�2��0.1mol/LFeCl3��Һ�������ɺ��ɫ���� | ֤������ͬ�¶��£��ܽ�ȣ�Mg��OH��2��Fe��OH��3 |
| B | �����ݵ������Ũ�ȵ�H2O2��Һ�У��ֱ�����Ũ�ȵ����CuSO4��KMnO4��Һ�����߲�������϶� | ֤��KMnO4��Һ�Ĵ�Ч�ʸ��� |
| C | �ⶨ��ͬ���ʵ���Ũ�ȵĹ����� ��̼������Һ��PH��ǰ�߽ϴ� | ֤���ǽ����ԣ�C��Si |
| D | ��FeCl3��Һ�м�����������Һ��ɫ��dz | ֤��FeCl3��Һ�д���ˮ��ƽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�������ɫ��������������ֲ��� | |
| B�� | ��ҺŨ�Ⱥ�pHֵ�����ֲ��䣬��Һ�²���1.73g������������ | |
| C�� | ��Һ�б����������������ӵ���Ŀ�����ֲ��䣬${\;}^{1{8}^{\;}}$O��������Һ�����У�������������1.73�� | |
| D�� | ��Һ�б����������������ӵ����ʵ�����Ũ�ȱ��ֲ��䣬${\;}^{1{8}^{\;}}$O��������Һ�����У�������������1.73�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
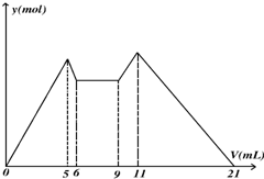
| A�� | ԭ���Һ�У�c��Al3+����c��Mg2+����c��Cl-��=1��1��5 | |
| B�� | A��NaOH��B�����ᣬ��c��NaOH����c��HCl��=2��1 | |
| C�� | ��A��B��ΪһԪǿ�����һԪǿ���V��A����V��B��=7��13 | |
| D�� | ��6��9����Ӧ���ӷ�ӦʽH++OH-�TH2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ԭ��Ӧ | B�� | ������ԭ��Ӧ��Ԫ����ͬ | ||
| C�� | ����������Ӧ��Ԫ�ز�ͬ | D�� | ����KCl�����ʵ���Ϊ2��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com