
µŖŹĒµŲĒņÉĻ¼«ĪŖ·įø»µÄŌŖĖŲ”£
£Ø1£©N2ŹĒ“óĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»£¬ÓÉÓŚ·Ö×ÓÖŠ¼üÄÜŗÜ“ó£¬ĖłŅŌŠŌÖŹĪČ¶Ø”£ŅŃÖŖN”ŌNµÄ¼üÄÜĪŖ946 kJ”¤mol£1£¬N”ŖNµ„¼üµÄ¼üÄÜĪŖ193 kJ”¤mol£1”£
¼ĘĖć£ŗN2·Ö×ÓÖŠ”°¦Š”±¼üµÄ¼üÄÜŌ¼ĪŖ £»
½įĀŪ£ŗN2·Ö×ÓÖŠ”°¦Ņ”±ŗĶ”°¦Š”±¼üµÄĪČ¶ØŠŌ ”£
£Ø2£©µŖµÄŃõ»ÆĪļŹĒ“óĘųĪŪČ¾ĪļÖ®Ņ»”£ĪŖĮĖĻū³żĪŪČ¾£¬æĘŃŠČĖŌ±Éč¼ĘĮĖĶ¬Ź±Ļū³ż¶žŃõ»ÆĮņŗĶµŖµÄŃõ»ÆĪļµÄ·½·Ø£¬Ę乤ŅÕĮ÷³ĢČēĻĀ£ŗ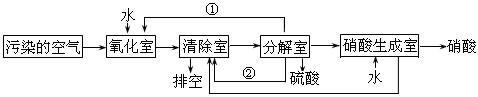
ĘäÖŠĒå³żŹŅ”¢·Ö½āŹŅ·¢ÉśµÄ·“Ó¦ČēĻĀ£ŗ
Ēå³żŹŅ£ŗNO + NO2 = N2O3 N2O3 + 2H2SO4 = 2NOHSO4 + H2O
·Ö½āŹŅ£ŗ4NOHSO4 + O2 + 2H2O = 4H2SO4 + 4NO2
»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ń£®¢ŁŗĶ¢Ś·Ö±šĪŖ£ØŠ“»ÆѧŹ½£© ”¢ £»
¢ņ£®Ńõ»ÆŹŅ·¢ÉśµÄ·“Ó¦ŹĒ £»
£Ø3£©½šŹōµŖ»ÆĪļŹĒŅ»ĄąÖŲŅŖµÄ»ÆѧĪļÖŹ£¬ÓŠ×ÅĢŲŹāµÄÓĆĶ¾”£Ä³½šŹōĄė×Ó£ØM+£©ÓėN3”ŖŠĪ³ÉµÄ¾§Ģå½į¹¹ČēÓŅĶ¼ĖłŹ¾”£Ęä ÖŠM+ÖŠĖłÓŠµē×ÓÕżŗĆ³äĀśK”¢L”¢MČżøöµē×Ó²ć£¬ĖüM+µÄ·ūŗÅŹĒ £¬ÓėĶ¬Ņ»øöN3£ĻąĮ¬µÄM+ÓŠ øö”£
ÖŠM+ÖŠĖłÓŠµē×ÓÕżŗĆ³äĀśK”¢L”¢MČżøöµē×Ó²ć£¬ĖüM+µÄ·ūŗÅŹĒ £¬ÓėĶ¬Ņ»øöN3£ĻąĮ¬µÄM+ÓŠ øö”£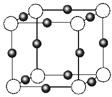
£Ø4£©NH3¼ČŹĒÖŲŅŖµÄ¹¤Ņµ²śĘ·£¬ÓÖŹĒÖ÷ŅŖµÄ¹¤ŅµŌĮĻ”£ŅŌNH3ĪŖŌĮĻÉś²śĻõĖįļ§µÄ¹ż
³ĢČēĻĀ£ŗ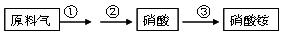
ĘäÖŠ·“Ó¦¢ŚĪŖ£ŗ4NO+3O2+2H2O=4HNO3 ŌĮĻĘųĪŖ°±ĘųŗĶæÕĘųµÄ»ģŗĻĪļ£¬¼ŁÉčæÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹżĪŖ0.2”£
¢ń£®Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ ”£Čō²»æ¼ĀĒø±·“Ó¦ĒŅø÷²½·“Ó¦¾łĶźČ«£¬Éś²ś¹ż³ĢÖŠŌĮĻĘųÖŠµÄ°±Ęų£Ø²»°üŗ¬µŚ¢Ū²½±»ĻõĖįĪüŹÕµÄ°±Ęų£©ŗĶæÕĘųÖŠŃõĘųĒ”ŗĆČ«²æ×Ŗ»ÆĪŖĻõĖį£¬ŌņŌĮĻĘųÖŠÖʱøĻõĖįµÄ°±ĘųŗĶŃõĘųµÄĢå»ż±ČĪŖ ”£
¢ņ£®ČōŹµ¼ŹÉś²śÖŠ£¬·“Ó¦¢ŁÖŠ°±µÄ×Ŗ»ÆĀŹ£Ø»ņĄūÓĆĀŹ£©ĪŖ70%£¬·“Ó¦¢ŚÖŠNOµÄ×Ŗ»ÆĀŹĪŖ90%£¬·“Ó¦¢ŪÖŠ°±ŗĶĻõĖį¾łĶźČ«×Ŗ»Æ”£ŌņÉś²śĻõĖįµÄ°±ĘųÕ¼ĖłÓĆ°±Ęų×ÜĮæµÄĢå»ż·ÖŹżĪŖ¶ąÉŁ£æ£ØŠ“³ö¼ĘĖć¹ż³Ģ£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ӣ
Ӣ
 Ӣ
Ӣ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| »Æѧ¼ü | N-N | N=N | N”ŌN | N-H | H-H |
| ¼üÄÜ/kJ?mol-1 | 159 | 418 | 946 | 391 | 436 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com