
����Ŀ��������ijɷ���ҪΪFeCr2O4������������Al2O3��SiO2���Ӹ���������ȡ�������Ļ�����,����ø�����Ĺ�������������
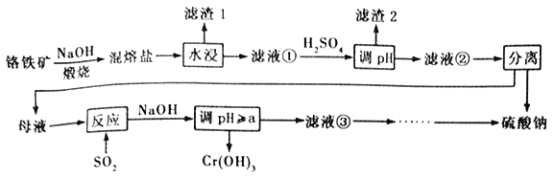
��֪�������Ρ��к���Na2CrO4��NaFeO2������NaFeO2����ˮ�⡣�Իش�����������
��1��������1��Ϊ���ɫ�������仯ѧʽΪ_________________������Һ���м�H2SO4����pH����Ŀ����һ��ʹCrO42-ת��ΪCr2O72- ������____________________________��
��2�������ա�������ʱ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��3��������ͼ�ܽ��(S)~�¶�(T)�����ж�������Һ�����е���ѡ����롱����Ϊ_________(�����)��
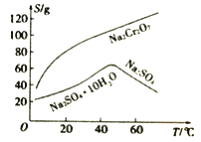
A.�����ᾧ B.����Ũ�������ȹ��� C.����Ũ������ȴ�ᾧ������
��4������Ӧ�������ӷ���ʽΪ_______________________________��
��5��Cr(OH)3������������������[CrCln(H2O) 6-n]x+��0.0015 mol [CrCln(H2O) 6-n]x+�������ӽ�����֬(HR)��ȫ�������ӽ�����
[CrCln(H2O) 6-n]x++xHR��Rx[CrCln(H2O) 6-n]+xH+�����ɵ�H+����25.00 mL0.1200 mol��L -1NaOH��Һǡ���к͡���������ӵĻ�ѧʽΪ__________________��
��6����֪25��ʱ��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]=1.0��10-32����ʹCr3+ǡ����ȫ����������Һ��c(Cr3+) =1.0��10-5 mol��L -1ʱ��Ӧ������ҺpH����СֵaΪ����? (��ʽ����)____________________________________________��
���𰸡� Fe(OH)3 ʹAlO2-��SiO32-ת��Ϊ���������ڹ��˷��� 4FeCr2O4��20NaOH��7O2![]() 8Na2CrO4��4NaFeO2��10H2O B 3SO2��Cr2O72-��2H+===2Cr3��+3SO42-+H2O [CrCl(H2O)5]2+ Cr3+��ȫ����ʱ��c(Cr3��)��1.0��10-5 mol��L-1��c(OH-)��1.0��10-9 mol��L-1 ��c(H��)��1.0��10-5 mol��L-1 ��pH��-lg 10-5��5
8Na2CrO4��4NaFeO2��10H2O B 3SO2��Cr2O72-��2H+===2Cr3��+3SO42-+H2O [CrCl(H2O)5]2+ Cr3+��ȫ����ʱ��c(Cr3��)��1.0��10-5 mol��L-1��c(OH-)��1.0��10-9 mol��L-1 ��c(H��)��1.0��10-5 mol��L-1 ��pH��-lg 10-5��5
��������������ijɷ���ҪΪFeCr2O4(��������Al2O3��SiO2)���������������պ����ɵ������������к���Na2CrO4��NaFeO2��ˮϴ�����Һ�к���Na2CrO4��NaFeO2���Լ���Ӧ���ɵ�ƫ�����ƺ����ƣ�������1��Ϊ���ɫ��������Ҫ�ɷ�Ϊ������������Һ1�м������ᣬ����pH��CrO42-ת��ΪCr2O72-��ƫ�����ƺ�����ת��Ϊ���������������������NaFeO2����ˮ��ת��Ϊ����������������Һ2����Ҫ�����ظ����ƺ������ƣ�ͨ�����������ظ����ƻ�ԭΪCr3�����ټ����������Ƶ���pH������Cr(OH)3��
(1)��������������������1��Ϊ���ɫ����Ϊ�����������仯ѧʽΪFe(OH)3������Һ���м�H2SO4����pH����Ŀ�ģ�һ��ʹCrO42-ת��ΪCr2O72- ������ʹAlO2-��SiO32-ת��Ϊ���������ڹ��˷��룬�ʴ�Ϊ��Fe(OH)3��ʹAlO2-��SiO32-ת��Ϊ���������ڹ��˷��룻
(2)��������������ʱ��FeCr2O4���������Ʒ�Ӧ������Na2CrO4��NaFeO2����Ӧ�Ļ�ѧ����ʽΪ4FeCr2O4��20NaOH��7O2![]() 8Na2CrO4��4NaFeO2��10H2O���ʴ�Ϊ��4FeCr2O4��20NaOH��7O2
8Na2CrO4��4NaFeO2��10H2O���ʴ�Ϊ��4FeCr2O4��20NaOH��7O2![]() 8Na2CrO4��4NaFeO2��10H2O��
8Na2CrO4��4NaFeO2��10H2O��
(3)��Һ2����Ҫ�����ظ����ƺ������ƣ������ܽ��(S)~�¶�(T)���߿�֪������Һ�ڽ��е����������������Ϊ����Ũ�������¶Ƚϸ�ʱ���ȹ��ˣ���ȥ�����ƣ��ʴ�Ϊ��B��
(4)����Ӧ��ʱ����������ظ����ƻ�ԭΪCr3�������ӷ���ʽΪ3SO2��Cr2O72-��2H+==2Cr3��+3SO42-+H2O���ʴ�Ϊ��3SO2��Cr2O72-��2H+==2Cr3��+3SO42-+H2O��
(5)[CrCln(H2O) 6-n]x++xHR��Rx[CrCln(H2O) 6-n]+xH+�����ɵ�H+����25.00 mL0.1200 mol��L -1NaOH��Һǡ���кͣ���Ϊn(OH-)=0.025L��0.1200 mol��L -1=0.003mol����n(H��)��0.0015 mol������[CrCln(H2O) 6-n]x++xHR��Rx[CrCln(H2O) 6-n]+xH+��֪��0.0015 mol [CrCln(H2O) 6-n]x+����0.003 mol H����x=2�������������ϼ۵Ĵ�����Ϊ0��[CrCln(H2O) 6-n]2+��CrΪ+3�ۣ�ClΪ-1�ۣ���n=1����˸������ӵĻ�ѧʽΪ[CrCl(H2O)5]2+���ʴ�Ϊ��[CrCl(H2O)5]2+��
(6)Cr3+��ȫ����ʱ��c(Cr3��)��1.0��10-5 mol��L-1��c(OH-)��![]() =
=![]() =1.0��10-9 mol��L-1 ��c(H��)��1.0��10-5 mol��L-1 ��pH��-lg 10-5��5���ʴ�Ϊ��5��
=1.0��10-9 mol��L-1 ��c(H��)��1.0��10-5 mol��L-1 ��pH��-lg 10-5��5���ʴ�Ϊ��5��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�ʻ���(COS)����ΪѬ���������ӽṹ��CO2�������ش��������⣺
��1��̼ԭ�ӵĺ�������Ų�ʽΪ____________������ʻ����Ԫ���У��뾶����ԭ���������_____�ֲ�ͬ�����ĵ��ӡ�
��2���ʻ���Ϊ___(���������������Ǽ�����)���ӣ��ʻ���ĵ���ʽΪ_____________��
��3�������ȶ���CO2����CS2��ԭ��________________________________________��
II.�ܱ������У�������Ӧ��CO(g)+H2S(g)![]() COS(g)+H2(g)
COS(g)+H2(g)
��4����֪�淴Ӧ������ʱ��仯��ͼ��ʾ����t0ʱ�ı������������_____________��________________��

��5���÷�Ӧ�ﵽƽ��������������䣬�����¶ȣ�H2SŨ�����ӣ������÷�Ӧ��___���������������������ȷ�Ӧ��ƽ�ⳣ��K��____(����������������С������������)�����ڷ�Ӧ��ϵ��ͨ��һ����SO2(g)���ж�ƽ���ƶ��ķ�����ԭ�� ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
A. C��H��O��N��Fe��P B. H��O��K��S��P��Mg
C. C��P��O��S��H��N D. N��P��K��Ca��S��Zn
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���ˮ�����ε�������,�������
A. ������ϸ������Դ����֮һ B. ˮ��ά��ϸ����̬������Ҫ����
C. ijЩ���β��빹��ϸ�����ӻ����� D. ˮ�ڻ�ϸ������Ҫ������ˮ��ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ�����(POCl3)�������뵼����Ӽ���ʵ������ȡPOCl3���ⶨ��Ʒ������ʵ��������£��ݴ˻ش�����������
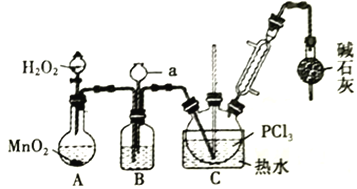
��.����Һ̬PCl3���Ʊ�POCl3��
����i���й��Լ������ʼ�ʵ��װ�������ȼ��г�װ��ʡ����������
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | ���߾�Ϊ��ɫҺ�壬��ܽ⣻��ˮ������ˮ��Ϊ�����ᡣ |
POCl3 | 2.0 | 106.0 | 153.5 |
��1��A �з�Ӧʱ��MnO2��������_________________������a������Ϊ_________________��
��2��ʢ��Ũ�����װ��B�������ó��۲�O2������֮��������_________________��
��3��ʵ��ʱӦ���Ʒ�Ӧ�¶���60~65�����¶Ȳ��˹��ߵ�ԭ����_________________��;�����ȥװ�м�ʯ�ҵĸ����������ܽ���POCl3�IJ�����ԭ����_________________________________(�û�ѧ����ʽ��ʾ����дһ��)��
��.�����ζ����ⶨPOCl3������
����ii ��Ag3PO4Ϊ��ɫ������������������
iii��Ag++SCN-=AgSCN������Ksp(AgSCN) <Ksp(AgCl)��
ʵ�鲽��������
����������ƿ�еIJ�ƷPOCl3ȥ������������60.00mL����ˮ��������ʹ����ȫˮ������ˮ��Һ���100.00mL��Һ��
��ȡ10.00mL��Һ����ƿ�У�����10.00mL 3.8mol/L AgNO3����Һ��
����������������������ҡ����ʹ�������渲����������
������2~3 ����������Һ��ָʾ������0.2mol/L KSCN��Һ�ζ�������AgNO3��Һ�������յ�ʱ����ȥ10,00mL KSCN��Һ��
��4���ﵽ�յ�ʱ��������______________________________��
��5�����ò�ƷPOCl3������Ϊ____________(���������λС��)����ȡ���������������ý����______________��� ƫ�ߡ����� ƫ�͡����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�����ⶨij�Ѳ��ֱ��ʵ�С�մ���Ʒ��Na2CO3�������������������ʵ�鷽����
������һ����ȡһ��������Ʒ�����������м��������غ�����ȴ������ʣ��������������㡣
��1�������з�����Ӧ�Ļ�ѧ����ʽΪ_______________��
��2��ʵ���У�����������ص�Ŀ����_______________��
������������ȡһ��������Ʒ������С�ձ��У�������ˮ�ܽ�����С�ձ��м�������Ba(OH)2��Һ��������ϴ�ӡ���������������������������㡣(��֪:Ba2++OH-+HCO3-==BaCO3��+H2O)
��1�����˲������������ձ���©��������Ҫ�õ��IJ�������Ϊ_______________��
��3��ʵ�����жϳ����Ƿ���ȫ�ķ�����_______________��
����������������ͼ��ʾװ�ý���ʵ��:
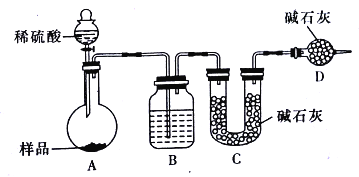
��1��Dװ�õ�������_______________����Һ©����_________(������������������)���������ϡ�������ʵ�顣
��2��ʵ��ǰ��ȡ17.90g��Ʒ��ʵ�����Cװ������8.80g������Ʒ��Na2CO3����������Ϊ________________(������λ��Ч����)
��3�����ݴ�ʵ���õ����ݣ��ⶨ������������Ϊʵ��װ�û������һ������ȱ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺ I���������ʵ�����A��B��Ϸ���2L���ܱ������У�������Ӧ3A��g��+B��g��xC��g��+2D��g������5min��ﵽƽ�⣬ƽ��ʱ���D��Ũ��Ϊ0.5mol/L��c��A����c��B��=3��5��v��C��=0.1mol/��Lmin������
��1��x= ��
��2��ǰ5min��B�ķ�Ӧ����v��B��= ��
��3��ƽ��ʱA��ת����Ϊ ��
��4��II��ˮ����ͨ�����ȵ�̼�㷢����Ӧ��C��s��+H2O��g��CO��g��+H2��g����H�� ��֪��K��300�棩��K��350�棩����÷�Ӧ���ȷ�Ӧ��
��5��������Ӧ��t0ʱ�̴ﵽƽ�⣬��t1ʱ�̸ı�ijһ����������Ӧ���ʣ���������ʱ��ı仯����ͼ��ʾ�������Ӧ�ı�ţ� 
����С��������� �ڽ����¶�
��6����֪��Ӧ��CO��g��+CuO��g��CO2��g��+Cu��s����H2��g��+CuO��g��Cu��s��+H2O��g�� ����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2 �� ���¶��·�ӦCO��g��+H2O��g��CO2��g��+H2��g����ƽ�ⳣ��K=����K1��K2��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���û�ѧ��Ӧԭ���о������仯���������ش���ش���������:
��1����֪: N2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4 kJ/mol
2NH3(g) ��H=-92.4 kJ/mol
N2(g)+O2(g)=2NO(g) ��H=+180.5 kJ/mol
2H2(g)+O2(g)=2H2O(g) ��H=-483.6kJ/mol
����34g��������������ȫ����һ�����������ˮ�������ų�������Ϊ_______��
��2��T1�¶�ʱ�����ݻ�Ϊ2 L �ĺ����ܱ������з�����Ӧ: 2NO(g)+O2(g)![]() 2NO2(g) ��H<0��ʵ����:v��=v(NO)����=2v(O2 )����=k��c2(NO)��c(O2)��v��=v(NO2)����=k��c2(NO2) ��k����k��Ϊ���ʳ��������¶�Ӱ�졣�����и���Ӧ�������������ʵ�����ʱ��仯��ͼ��ʾ:
2NO2(g) ��H<0��ʵ����:v��=v(NO)����=2v(O2 )����=k��c2(NO)��c(O2)��v��=v(NO2)����=k��c2(NO2) ��k����k��Ϊ���ʳ��������¶�Ӱ�졣�����и���Ӧ�������������ʵ�����ʱ��仯��ͼ��ʾ:
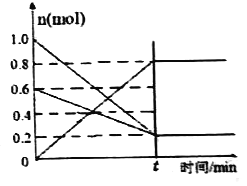
������˵���ܱ����÷�Ӧ�Ѵﵽƽ��״̬����______(�����)��
A.���������ܶȲ��� B.����������ɫ����
C. k����k������ D.2v��(O2)= v��(NO2)
����ѧƽ�ⳣ��K�����ʳ���k����k������ѧ��ϵ��K=_______��
��T1�¶�ʱ����ѧƽ�ⳣ��K=______�����������¶ȸı�ΪT2ʱ����k��=k�� ����T2___T1 (����>������<������=��)��
��3�� �������ǵ�Ԫ����Ҫ�ĺ�����֮һ��25�棬���amol/LHNO2ϡ��Һ��pH=b�����¶���HNO2����ƽ�ⳣ���ľ�ȷ�������ʽΪK=______(�ú�a��b�Ĵ���ʽ��ʾ)����amol/L��NaCN��Һ��0.01mol/L������������Ϻ����ҺpH=7������Һ��![]() =_____(�ú�a �Ĵ���ʽ��ʾ)��
=_____(�ú�a �Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Թ�A���ȼ���3 mL��ˮ�Ҵ���Ȼ������Թܱ���������2 mLŨ���ᣬ������2 mL�����ᣬ����ͼװ�ý���ʵ�飬��������������������ͨ���Թ�B�б���̼������Һ��Һ���ϡ���ش��������⣺

��1��Ũ�����������__________________________________________��
��2���Թ�B�й۲쵽��������_____________________________��
��3�����Թ�B�еı���̼������Һ����ˮ���棬ʵ����������ŵ���ζ�Ŀ���ԭ����________________________________________________________________________��
��4���Թ�B�еı���̼������Һ��������__________________________________��
��5��ͨ�������ĵ��ܲ��ܲ����Թ�B��Һ���µ�ԭ����_______________________________________��
��6��ͨ��������ѡ�ó����ܵ�Ŀ����________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com