
Ϊ̽����ҵ����������ͭ�Ͻ���ϵ������ã���ͬѧ��Ƶ�ʵ�鷽�����£�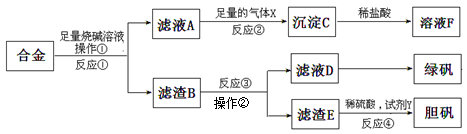
��ش�
��1���̷��Ļ�ѧʽΪ ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ����Ӧ�����ɳ��������ӷ�Ӧ����ʽ ��
��3��Ϊ�˼����ҺD�к��еĽ������ӣ������ʵ�鷽��Ϊ���Լ���ѡ���� ��
��4��������B�еμ�ϡ����ʱ�����ַ�Ӧ���ʱ�һ������۷�ӦҪ�죬��ԭ���� ��
��5����������ɫ��ѧ���գ�������E�м���ϡ������Լ�Y�Ƶ������壬�Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽΪ ������������ɫ��ѧ���գ���ѡ�Լ�YΪ1mol/L�����ᣬ��ʹ3molCuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4��������������� L��
��1��(2��) FeSO4��7H2O
��2����4�֣�2Al+2NaOH+2H2O=2NaAlO2+3H2�� AlO2-+CO2+2H2O=Al(OH)3��+HCO3-
(��2AlO2-+CO2+3H2O=2Al(OH)3��+CO32-) [δд���ӷ���ʽ��1�֣�����ƽ��1�֣����ӷ��Ŵ�0��]
��3��(3��) ���Թ�ȡ������ҺD��1�֣�������Һ�еμ�KSCN����NaSCN����NH4SCN)]
��Һ����������1�֣����ٵ�����ˮ����˫��ˮ����ͨ��Cl2�������Ѫ��ɫ������
Һ���д���Fe2+��1�֣���
��4����2�֣�ͭ������ϡ�����γ���ԭ��أ������˵绯��ʴ�����е���������
��5��(5��) Cu+H2O2+H2SO4=CuSO4+2H2O��2�֡���ƽ1�֣���ѧʽ��0�֣�
2��3�֣�[˵������ӦʽΪ3Cu+2HNO3+3H2SO4=3CuSO4++2NO��+4H2O]
���������������1���̷�Ϊ�����������壬��ѧʽΪ��FeSO4��7H2O
��2����Ӧ��ΪAl��NaOH��Һ�ķ�Ӧ�����ӷ���ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2������Ӧ����ͨ�����������XΪCO2��CO2��H2O��AlO2-��Ӧ���ɵij���ΪAl(OH)3�����ӷ���ʽΪ��AlO2-+CO2+2H2O=Al(OH)3��+HCO3- (��2AlO2-+CO2+3H2O=2Al(OH)3��+CO32-)
��3����ҺD������ΪFeSO4������Fe2+��ԭ��Ϊ��Fe2+����ʹKSCN��ΪѪ��ɫ����������������Fe2+����ΪFe3+����Һ��ΪѪ��ɫ������ʵ�鷽��Ϊ�����Թ�ȡ������ҺD������Һ�еμ�KSCN����NaSCN����NH4SCN)]����Һ�����������ٵ�����ˮ����˫��ˮ����ͨ��Cl2�������Ѫ��ɫ������Һ���д���Fe2+��
��4������B�к�������ͭ�������μ�ϡ�����ͭ������ϡ�����γ���ԭ��أ�ʹ��Ӧ���ʼӿ졣
��5������EΪCu������ϡ������Լ�Y����CuSO4��������ɫ��ѧ���գ�YΪ��ɫҺ�壬���Լ�YΪH2O2����Ӧ�ܵ��ܻ�ѧ����ʽΪ��Cu+H2O2+H2SO4=CuSO4+2H2O��Cuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4����ѧ����ʽΪ��3Cu+2HNO3+3H2SO4=3CuSO4++2NO��+4H2O��CuΪ3mol�������ĵ�HNO3Ϊ2mol�������������Ϊ��2mol��1mol?L?1=2L��
���㣺���⿼�黯ѧ���̵ķ�������ѧ����ʽ�����ӷ���ʽ����д�����ӵļ��顢ԭ���ԭ������ѧ���㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ������ת����ϵ�У���Ӧ�����Ͳ���������δȫ���г�����X���ʿ��������ں����У�A��BΪ�������嵥�ʣ�BΪ����ɫ���壬I��LΪ�����Ľ������ʣ�GΪ���ɫ���ʡ���ش��������⣺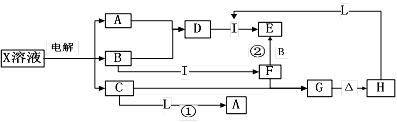
��1��X��ѧʽΪ �� ��2��C��ѧʽΪ ��
��3����Ӧ�ٵ����ӷ���ʽΪ ��
��4����Ӧ�ڵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�����ͭ�ڻ�����ũҵ�����кܹ㷺���ô���ij��ѧ��ȤС��������ϣ������ֲ�ͬ��ԭ����ȡ����ͭ��
��ʽһ��һ�ֺ�ͭ�Ŀ�ʯ�����ȸʯ��ۣ�����ͭ��̬ΪCuCO3��Cu(OH)2��CuSiO3��2H2O������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��������ֿ�ʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��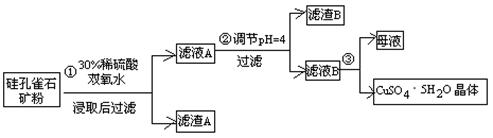
��ش��������⣺
����ɲ������ϡ������CuSiO3��2H2O������Ӧ�Ļ�ѧ����ʽ
CuSiO3��2H2O+H2SO4=CuSO4 +________+H2O��
�Ʋ���ڵ�����ҺpHѡ�õ�����Լ���__________________
A. CuO B. MgO C. FeCO3 D NH3��H2O
���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�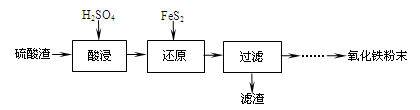
��1���������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ��� ��
��2������ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ
��
��3��Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��
ȷ��ȡһ���������������Һ����ƿ�У�����HCl���Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ����ʽ���£�
2Fe3����Sn2����6Cl����2Fe2����SnCl62����
Sn2����4Cl����2HgCl2��SnCl62����Hg2Cl2����
6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O��
����SnCl2����������ⶨ��Fe3���� (�ƫ�ߡ�����ƫ�͡��������䡱����ͬ)��
��������HgCl2����ⶨ��Fe3���� ��
��4���ٿ�ѡ�� (���Լ�)������Һ�к���Fe3+������Fe3+��ԭ����
(�����ӷ�Ӧ����ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��м�����ڵ���ˮ�ѵ�����ʵ�����о��������£�
����м������0.5 mol/L�����н���Ԥ������
��30 min����ȥ����ˮ������ϴ������ϴ��Һ���pHΪ���ԡ���N2�����º�ɱ��á�
��������ˮ�м�������������������Һ��
��������Ԥ���������м�����������Һ�С�
��ش�
��1�������ܽ�Fe2O3�����ӷ���ʽ�� ��
��2�����ʱ��Ҫ��N2�����½��е�ԭ���� ��
��3����������г�ϴ�����Һ�ڿ����м����������գ����յõ��Ĺ����� ��
��4�������������£�����NO3����Ӧ�����ӷ���ʽ����������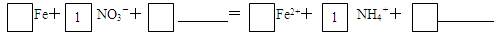
��5����֪����̿������NH4�� ��OH����������в���ʱ����м�ͻ���̿ͬʱ�����������Һ�У���������ѵ���Ч������ԭ���� ��
��6���о�������ҺpH��Ӱ����м�ѵ���Ч������Ӧ��ϵ��pH�ֱ������4 ��8. 5 ʱ��NO3����ȥ���ʷֱ�Ϊ90% ��15%����������ˮ�к���CO32������Ӱ���ѵ���Ч�����û�ѧ��������ּ�����ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A���ĩ������B��ɵĻ�����ܷ�����ͼ��ʾ��һϵ�з�Ӧ��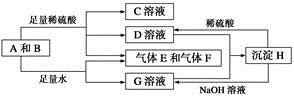
��ش��������⣺
(1)���A���ʵ�Ԫ�������ڱ��д��ڵ�__________����__________�塣
(2)������B�ĵ���ʽΪ_____________________________��
(3)D��G����Һ��Ϻ�����Ӧ�����ӷ���ʽΪ____________________
(4)�����£�D��Һ��pH________7(�����������������)����ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(5)10.8 g A������������NaOH��Һ��Ӧ������������������Ϊ________ g��
(6)��̼����ϡ���ᡢ����E������F���ȼ�ϵ�أ��õ�ص�������ӦʽΪ______________________���Ըõ��Ϊ��Դ���ö��Ե缫���100 g 8%��C��Һ�������ʵ���������Ϊ12.5%ʱֹͣ��⣬��������У����ɵ�������״���µ������Ϊ________ L����·��ͨ�����ӵ����ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��I��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ��������Ҫ�����ɵ�ˮ���������ֲ�������ȥ����ش��������⣺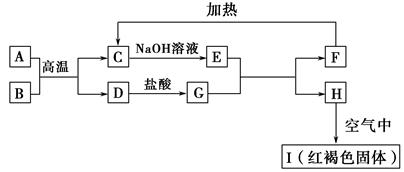
(1)B��F�ֱ��� (�ѧʽ)��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��A��B�ڸ����·�Ӧ�� ��
��H�ڿ�����ת��ΪI�� ��
(3)E��Һ����������Ũ���ɴ�С��˳���� ��
(4)�����ӷ�Ӧ����ʽ��ʾG��Һ�����Ե�ԭ�� ���÷�Ӧ��ƽ�ⳣ��Ϊ (��֪�����£�H���ܶȻ�����Ksp��8.0��10��16)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1862�꣬����ʱ��ѧ������ά�����˰���Ƽ1926�꣬�ҹ���ѧ�Һ�°�����
��Ϊ����°��Ƽ��Ҳ�������Ƽ�������Ƽ���������̿ɼ�Ҫ��ʾ����ͼ��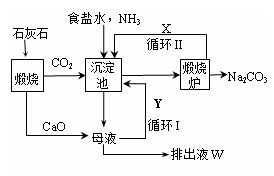
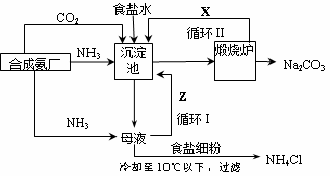
����������� �����Ƽ��������
��1�����������ͨ��CO2�Ͱ���ʱ��Ӧ��ͨ�백����ԭ���� ��
��2���������з�����Ӧ�Ļ�ѧ��Ӧ����ʽ�� �ӳ������з�������IJ����� ��
��3�������������ʾ��ͼ�е�Y�� ����ԭ�ϵ���Ʒ������ܷ�Ӧ�����û�ѧ����ʽ��ʾ����дΪ ��
��4�������Ƽ�д���Һ����ȡ�Ȼ�茶���Ĺ����Ʋ⣬���ý�����ȷ�� ��ѡ���ţ���
a������ʱ�Ȼ�淋��ܽ�ȱ��Ȼ���С
b��ͨ�백��������NH4+��Ũ�ȣ�ʹ�Ȼ�笠�������
c������ʳ��ϸ�������Na+��Ũ�ȣ� ʹNaHCO3�ᾧ����
d��ͨ�백����ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
��5�������Ƽ����ڰ�����Ȼ��������ʴ�70%��ߵ�90%���ϣ���Ҫ�������ѭ���������Ƽ����һ���ŵ��� ��
��6����Ʒ�����к���̼�����ƣ������ü��ȷֽ�ķ����ⶨ��Ʒ�д����������������֪��Ʒ����Ϊag���������������ٸı�ʱ����Ϊbg�������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�մ��С�մ����Ź㷺��Ӧ�á��Դӷ�Ӧ�������Ƕ�˵����������;��ѡA(�մ�)����B(С�մ�)����˵��ԭ��
(1)������ʱ��ֹ��۽ϳ�ʱ�䴢�����ζ����������������ѡ��______����Ϊ______________________________
(2)��Ϊ��ĭ�������ҩƷ��ѡ��________����Ϊ________________________
(3)����ϴ�Ӳ;�ʵ���ҵIJ��������ȣ�ѡ��________����Ϊ_____________
(4)����θ�����ʱ��ѡ��________����Ϊ_______________________________
(��ʾ������ˮ��Һ���Լ��ԣ����մ�ļ���ǿ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com