
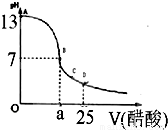
£Ø12·Ö£©Ä³Ń§ÉśŌŚŹµŃéŹŅ²ā¶ØŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį,ŅŃÖŖŌŚ25mlĒāŃõ»ÆÄʱź×¼ČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol/L“×ĖįČÜŅŗµÄPH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ŗ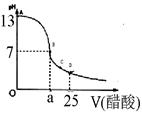
£Ø1£©øĆĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol.L”Ŗ1
£Ø2£©ŌŚBµć£¬a 12.5ml(Ģī”°>”±”¢”°<”±»ņ”°="”±" )”£
£Ø3£©ÅäÖĘ100 mL NaOH±ź×¼ČÜŅŗĖłŠčŅĒĘ÷³żĶŠÅĢĢģĘ½”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčŅŖ
£Ø4£©ÓĆ ĮæČ”20.00 mL“ż²āĻ”ŃĪĖįČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬²¢µĪ¼Ó2”«3µĪ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÓĆNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø”£ĪŖĮĖ¼õŠ”ŹµŃéĪó²ī£¬øĆĶ¬Ń§Ņ»¹²½ųŠŠĮĖČż“ĪŹµŃ飬¼ŁÉčĆæ“ĪĖłČ”Ļ”ŃĪĖįĢå»ż¾łĪŖ20.00 mL£¬Čż“ĪŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 19.00 | 23.00 | 23.02 |
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ä³Ń§ÉśŌŚŹµŃéŹŅ²ā¶ØŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį£¬ŅŃÖŖŌŚ25mlĒāŃõ»ÆÄʱź×¼ČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol/L“×ĖįČÜŅŗµÄPH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ŗ
Ä³Ń§ÉśŌŚŹµŃéŹŅ²ā¶ØŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį£¬ŅŃÖŖŌŚ25mlĒāŃõ»ÆÄʱź×¼ČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol/L“×ĖįČÜŅŗµÄPH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ŗ| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 19.00 | 23.00 | 23.02 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø12·Ö£©Ä³Ń§ÉśŌŚŹµŃéŹŅ²ā¶ØŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį,ŅŃÖŖŌŚ25mlĒāŃõ»ÆÄʱź×¼ČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol/L“×ĖįČÜŅŗµÄPH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ŗ
£Ø1£©øĆĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol.L”Ŗ1
£Ø2£©ŌŚBµć£¬a 12.5ml(Ģī”°>”±”¢”°<”±»ņ”°=”± )”£
£Ø3£©ÅäÖĘ100 mL NaOH±ź×¼ČÜŅŗĖłŠčŅĒĘ÷³żĶŠÅĢĢģĘ½”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčŅŖ
£Ø4£©ÓĆ ĮæČ”20.00 mL“ż²āĻ”ŃĪĖįČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬²¢µĪ¼Ó2”«3µĪ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÓĆNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø”£ĪŖĮĖ¼õŠ”ŹµŃéĪó²ī£¬øĆĶ¬Ń§Ņ»¹²½ųŠŠĮĖČż“ĪŹµŃ飬¼ŁÉčĆæ“ĪĖłČ”Ļ”ŃĪĖįĢå»ż¾łĪŖ20.00 mL£¬Čż“ĪŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 19.00 | 23.00 | 23.02 |
øĆŃĪĖįµÄÅضČŌ¼ĪŖ___________________(±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£
µĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾ŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ¶Č¹ć¶«Ź”ø߶žµŚŅ»Ń§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Ä³Ń§ÉśŌŚŹµŃéŹŅ²ā¶ØŅ»Ī“ÖŖÅØ¶ČµÄĻ”ŃĪĖį,ŅŃÖŖŌŚ25mlĒāŃõ»ÆÄʱź×¼ČÜŅŗÖŠÖšµĪ¼ÓČė0.2mol/L“×ĖįČÜŅŗµÄPH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾£ŗ
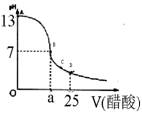
£Ø1£©øĆĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol.L”Ŗ1
£Ø2£©ŌŚBµć£¬a 12.5ml(Ģī”°>”±”¢”°<”±»ņ”°=”± )”£
£Ø3£©ÅäÖĘ100 mL NaOH±ź×¼ČÜŅŗĖłŠčŅĒĘ÷³żĶŠÅĢĢģĘ½”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčŅŖ
£Ø4£©ÓĆ ĮæČ”20.00 mL“ż²āĻ”ŃĪĖįČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬²¢µĪ¼Ó2”«3µĪ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ÓĆNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø”£ĪŖĮĖ¼õŠ”ŹµŃéĪó²ī£¬øĆĶ¬Ń§Ņ»¹²½ųŠŠĮĖČż“ĪŹµŃ飬¼ŁÉčĆæ“ĪĖłČ”Ļ”ŃĪĖįĢå»ż¾łĪŖ20.00 mL£¬Čż“ĪŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
|
ŹµŃé“ĪŹż |
µŚŅ»“Ī |
µŚ¶ž“Ī |
µŚČż“Ī |
|
ĻūŗÄNaOHČÜŅŗĢå»ż/mL |
19.00 |
23.00 |
23.02 |
øĆŃĪĖįµÄÅضČŌ¼ĪŖ___________________ (±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö)”£
µĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾ŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”Õ潶žÖŠø߶ž£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£ØĄķæĘ£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄNaOHČÜŅŗĢå»ż/mL | 19.00 | 23.00 | 23.02 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com