
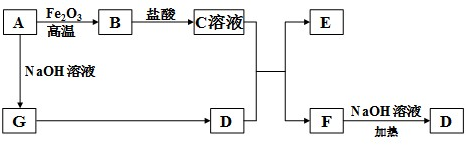
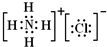 ���ʴ�Ϊ��
���ʴ�Ϊ��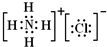 ��
��
| ||
| ||
| 5.4g |
| 27g/mol |
| ||
| ||


 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ȡ��������ȵ�CO��N2 |
| B�����µ������O2��N2 |
| C����������ܶȵ�CO��N2 |
| D����ѹ�������O2��N2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ϊ�Ƶ��ʣ���Ϊ����������һ����ˮ |
| B������Ϊ�����ʣ���Ϊ�����ʣ�����һ���������� |
| C������Ϊͭ���ʣ���Ϊ�Ȼ�������Һ������һ�����Ȼ�����Һ |
| D�����ס��ҡ���������Ϊ�������÷�Ӧһ�����ڸ��ֽⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| O2 |
| ����/�� |
| �Ҵ� |
| Ũ����/�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������� | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֬�������������Ǵ�����������¶���Һ�� |
| B����֬��������������ˮ����������ʹ� |
| C����֬����������������ʹ��ˮ��ɫ |
| D����֬������������������ˮ�����������л��ܼ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com