
ŃĒĀČĖįÄĘ£ØNaClO2£©Ö÷ŅŖÓĆÓŚĆŽ·Ä”¢ŌģÖ½ŅµµÄĘÆ°×¼Į£¬Ņ²ÓĆÓŚŹ³Ę·Ļū¶¾”¢Ė®“¦ĄķµČ£¬ŃĒĀČĖįÄĘŹÜČČŅ×·Ö½ā”£ŅŌĀČĖįÄʵČĪŖŌĮĻÖʱøŃĒĀČĖįÄĘµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ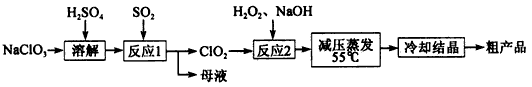
£Ø1£©Ģįøß”°·“Ó¦l”±·“Ó¦ĖŁĀŹµÄ“ėŹ©ÓŠ______________£ØŠ“³öŅ»Ģõ¼“æÉ£©”£
£Ø2£©”°·“Ó¦2”±µÄŃõ»Æ¼ĮŹĒ________£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________”£
£Ø3£©²ÉČ””°¼õŃ¹Õō·¢”±¶ų²»ÓĆ”°³£Ń¹Õō·¢”±£¬ŌŅņŹĒ__________________”£
£Ø4£©“Ó ”°ÄøŅŗ”±ÖŠæÉ»ŲŹÕµÄÖ÷ŅŖĪļÖŹŹĒ__________________________”£
£Ø5£©”°ĄäČ“½į¾§”±ŗó¾_____________£ØĢī²Ł×÷Ćū³Ę£©¼“æÉ»ńµĆ“Ö²śĘ·”£
£Ø12·Ö£©£Ø1£©ÉżøßĪĀ¶Č£¬Ōö“óĪüŹÕŅŗÅØ¶ČµČ”££Ø2·Ö£©
£Ø2£©ClO2£Ø2·Ö£© H2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2£Ø2·Ö£©
£Ø3£©³£Ń¹Õō·¢ĪĀ¶Č¹żøߣ¬ŃĒĀČĖįÄĘČŻŅ×·Ö½ā£»£Ø2·Ö£©
£Ø4£©Na2SO4£Ø2·Ö£©
£Ø5£©¹żĀĖ£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻĢõ¼ž¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ£¬ÅŠ¶ĻĢįøß”°·“Ó¦l”±·“Ó¦ĖŁĀŹµÄ“ėŹ©ÓŠÉżøßĪĀ¶Č£¬Ōö“óĪüŹÕŅŗÅØ¶ČµČ”£
£Ø2£©Ķعż·“Ó¦2µĆµ½ŃĒĀČĖįÄĘµÄ“Ö²śĘ·£¬ClO2ÖŠµÄClŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+4¼Ū£¬NaClO2ÖŠµÄClŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+2¼Ū£¬»ÆŗĻ¼Ū½µµĶ£¬ĖłŅŌ”°·“Ó¦2”±µÄŃõ»Æ¼ĮŹĒClO2£»Ōņ»¹Ō¼ĮŹĒ¹żŃõ»ÆĒā£¬±»Ńõ»ÆĪŖŃõĘų£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ H2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2£»
£Ø3£©ŃĒĀČĖįÄĘŹÜČČŅ×·Ö½ā£¬¼õŃ¹Õō·¢ŌŚ½ĻµĶĪĀ¶Č½ųŠŠ£¬¶ų³£Ń¹Õō·¢ĪĀ¶Č¹żøߣ¬ŃĒĀČĖįÄĘČŻŅ×·Ö½ā£¬ĖłŅŌ²ÉČ””°¼õŃ¹Õō·¢”±¶ų²»ÓĆ”°³£Ń¹Õō·¢”±£»
£Ø4£©ĀČĖįÄĘĖįČÜŗóÓė¶žŃõ»ÆĮņ·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉClO2,Ķ¬Ź±ÓŠNa2SO4Éś³É£¬ĖłŅŌ“Ó ”°ÄøŅŗ”±ÖŠæÉ»ŲŹÕµÄÖ÷ŅŖĪļÖŹŹĒNa2SO4£»
£Ø5£©ĄäČ“½į¾§ŗóŅŖ·ÖĄė³ö¹ĢĢ壬ӦÓĆ¹żĀĖµÄ·½·Ø”£
æ¼µć£ŗæ¼²éĪļÖŹµÄÖʱø£¬ĪļÖŹµÄ·ÖĄėĢį“æµÄ·½·Ø£¬»Æѧ·½³ĢŹ½µÄŹéŠ“



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ×°ÖĆ»ņ²Ł×÷ÓėŹµŃéÄæµÄ×īĻą·ūµÄŹĒ
| A£®¢Ł”ŖŹµŃéŹŅÖĘČ”²¢ŹÕ¼Æ×ćĮæNH3 |
| B£®¢Ś”ŖŅŌäå»Æ¼Ų”¢90%ĮņĖį”¢ŅŅ“¼ĪŖŌĮĻŗĻ³ÉäåŅŅĶéµÄ×°ÖĆ |
| C£®¢Ū”Ŗ¼ģ²é×°ÖĆĘųĆÜŠŌ |
| D£®¢Ü”ŖĄūÓĆÅÅæÕĘų·ØŹÕ¼ÆCO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÅäÖĘ500mL 0.1mol/LµÄNaOHČÜŅŗ£¬ÓŠČēĻĀ²Ł×÷²½Öč£ŗ
¢Ł¼ĘĖćĖłŠčNaOH¹ĢĢåµÄÖŹĮæ²¢ÓĆĶŠÅĢĢģĘ½³ĘČ”£»
¢Ś½«³ĘĮæŗƵÄNaOH¹ĢĢå·ÅČėÉÕ±ÖŠ£¬¼ÓČėŹŹĮæµÄÕōĮóĖ®Čܽā£¬Č»ŗó×ŖŅĘÖĮČŻĮæĘæÖŠ£»
¢ŪÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī£¬Ćæ“ĪĻ“µÓµÄŅŗĢ嶼Š”ŠÄ×ŖČėČŻĮæĘæÖŠ²¢ĒįĒįŅ”ŌČ£»
¢Ü¼ĢŠųĻņČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę¾ąæĢ¶ČĻß1”«2cm“¦£¬øÄÓĆ½ŗĶ·µĪ¹ÜŠ”ŠÄµĪ¼ÓÕōĮóĖ®ÖĮČÜŅŗ°¼ŅŗĆęÓėæĢ¶ČĻßĻąĒŠ£»
¢ŻČū½ōČŻĮæĘæµÄČū×Ó£¬³ä·ÖŅ”ŌČ”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČŻĮæĘæŌŚŹ¹ÓĆĒ°£¬±ŲŠė
£Ø2£©ŹµŃéÖŠÓĆĶŠÅĢĢģĘ½Źµ¼Ź³ĘČ”NaOH¹ĢĢåµÄÖŹĮæŹĒ
£Ø3£©ÉĻŹöŹµŃé²Ł×÷¢ŚÖŠ£¬Č±ÉŁµÄ²½ÖčŹĒ
£Ø4£©ŌŚŹµŃéÖŠ£¬Ī“½ųŠŠ²Ł×÷¢Ū£¬ĖłÅäČÜŅŗµÄÅØ¶Č»į £ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£¬ĻĀĶ¬£©£»¶ØČŻŹ±ø©ŹÓŅŗĆę£¬ĖłÅäČÜŅŗµÄÅØ¶Č»į ”£³ĘĮæĒ°ČŻĮæĘæÓŠÉŁĮæĖ®£¬ĖłÅäČÜŅŗµÄÅØ¶Č»į ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÖŲøõĖį¼ŲŹĒ¹¤ŅµÉś²śŗĶŹµŃéŹŅµÄÖŲŅŖŃõ»Æ¼Į£¬¹¤ŅµÉĻ³£ÓĆøõĢśæó£ØÖ÷ŅŖ³É·ÖĪŖFeO”¤Cr2O3£¬ŌÓÖŹĪŖSiO2”¢Al2O3£©ĪŖŌĮĻ²śĖü£¬ŹµŃéŹŅÄ£Äā¹¤Ņµ·ØÓĆøõĢśæóÖĘK2Cr2O7µÄÖ÷ŅŖ¹¤ŅÕČēĻĀĶ¼”£Éę¼°µÄÖ÷ŅŖ·“Ó¦ŹĒ6FeO”¤Cr2O3+24NaOH+7KClO3 12Na2CrO4+3Fe2O3+7KCl+12H2O”£
12Na2CrO4+3Fe2O3+7KCl+12H2Oӣ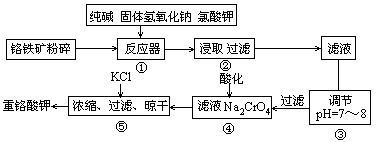
£Ø1£©¼ī½žĒ°½«ĆśĢśæó·ŪĖéµÄ×÷ÓĆŹĒ
£Ø2£©²½Öč¢Ūµ÷½ŚpHŗó¹żĀĖµĆµ½µÄĀĖŌüŹĒ ”£
£Ø3£©²Ł×÷¢ÜÖŠ£¬Ėį»ÆŹ±£¬CrO2- 4×Ŗ»ÆĪŖCr2O2- 7£¬Š“³öĘ½ŗā×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½
£»
£Ø4£©ÓĆ¼ņŅŖµÄĪÄ×ÖĖµĆ÷²Ł×÷¢Ż¼ÓČėKClµÄŌŅņ ”£
£Ø5£©³ĘČ”ÖŲøõĖį¼ŲŹŌŃł2.500gÅä³É250mLČÜŅŗ£¬Č”³ö25mLÓėµāĮæĘæÖŠ£¬¼ÓČė10mL2mol/ LH2SO4ŗĶ×ćĮæµā»Æ¼Ų£ØøõµÄ»¹Ō²śĪļĪŖCr3+)£¬·ÅÓŚ°µ“¦5min”£Č»ŗó¼ÓČė100mLĖ®£¬¼ÓČė3mLµķ·ŪÖøŹ¾¼Į£¬ÓĆ0.1200mol/LNa2S2O3±ź×¼ČÜŅŗµĪ¶Ø£ØI2+2S2O2- 3£½2I- +S4O2- 6£©
¢ŁÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ £»
¢ŚČōŹµŃéÖŠ¹²ÓĆČ„Na2S2O3±ź×¼ČÜŅŗ40.00mL£¬ŌņĖłµĆ²śĘ·ÖŠÖŲøõĖį¼ŲµÄ“æ¶ČĪŖ£ØÉčÕūøö¹ż³ĢÖŠĘäĖüŌÓÖŹ²»²Ī¼Ó·“Ó¦£© £Ø±£Įō2Ī»ÓŠŠ§Źż×Ö£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ĄūÓĆ·“Ó¦I2(s)+Cl2(g)=2ICl(l)£¬ŹµŃéŹŅæÉÓĆČēĻĀĶ¼ĖłŹ¾×°ÖĆ£Ø¼ÓČČ”¢¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£©ÖĘȔɣĮæIC1”£
ŅŃÖŖ£ŗIClµÄČŪµćĪŖ13.9”ę£¬·ŠµćĪŖ97.4”ę£¬Ņ×Ė®½ā£¬ĒŅÄÜ·¢Éś·“Ó¦£ŗ
ICl(l)+Cl2(g)=2ICl3(l)
£Ø1£©×°ÖĆAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________”£
£Ø2£©×°ÖĆBµÄ×÷ÓĆŹĒ______”£²»ÄÜÓĆ×°ÖĆF“śĢę×°ÖĆE£¬ĄķÓÉŹĒ____________”£
£Ø3£©ĖłÖʵƵÄIClÖŠČÜÓŠÉŁĮæICl3ŌÓÖŹ£¬Ģį“æµÄ·½·ØŹĒ______ (Ģī±źŗÅ£©”£
| A£®¹żĀĖ | B£®Õō·¢½į¾§ | C£®ÕōĮó | D£®·ÖŅŗ |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(15·Ö) £Ø1£©Ä³ŠĖȤŠ”×éÓū×¼Č·ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗ£ŗæģĖŁ×¼Č·³ĘČ”8.0 g “æ¾»µÄNaOH¹ĢĢ壬ÅäÖĘ³É100 mLČÜŅŗ£¬øĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ ”£ĖłŠčµÄŅĒĘ÷³żĶŠÅĢĢģĘ½”¢Ōæ³×”¢Š”ÉÕ±”¢²£Į§°ōĶā»¹Č±ÉŁ ”£
£Ø2£©ÓŠ»śĪļXÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³ÉµÄ£¬ĪŖČ·¶ØĘä½į¹¹½ųŠŠČēĻĀŹµŃé£ŗ
a£®6.0 g XŌŚŅ»¶ØĢõ¼žĻĀĶźČ«·Ö½ā£¬Éś³É3.36 L(±ź×¼×“æöĻĀ)Ņ»Ńõ»ÆĢ¼ŗĶ1.8 gĖ®£»
b£®ÖŠŗĶ2.4 g ÓŠ»śĪļXŠčĻūŗÄÉĻŹöNaOHČÜŅŗ20.00 mL£»
c£®0.1 molÓŠ»śĪļXĶźČ«×Ŗ»ÆĪŖõ„£¬ŠčŅŖŅŅ“¼9.2 g£¬0.1 mol XÄÜÓė×ćĮæÄĘ·“Ó¦·Å³ö3.36 L(±ź×¼×“æöĻĀ)ĒāĘų”£Ōņ£ŗ
¢ŁXµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ ”£
¢ŚXµÄ·Ö×ÓŹ½ĪŖ ”£
¢ŪŠ“³öXµÄ½į¹¹¼ņŹ½£ŗ ”£
¢ÜŠ“XÓė×ćĮæŅŅ“¼·¢Éśõ„»Æ·“Ó¦µÄ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(18·Ö)Įņ“śĮņĖįÄĘŌŚ¹¤ŅµÉś²śÖŠÓĆĶ¾·Ē³£¹ć·ŗ”£
£Ø1£©Na2S2O3ČÜŅŗŹĒ¶ØĮæŹµŃéÖŠµÄ³£ÓĆŹŌ¼Į”£ŹµŃéŹŅŠčÓĆ480mLŅ»¶ØÅØ¶ČµÄNa2S2O3ČÜŅŗ£¬ÅäÖĘøĆČÜŅŗĖłŠč²£Į§ŅĒĘ÷³żÉÕ±”¢ĮæĶ²”¢²£Į§°ōĶā£¬»¹Šč__________________”£
£Ø2£©Na2S2O3æÉŅŌÓĆ×÷Ēč»ÆĪļµÄ½ā¶¾¼Į£¬¹¤ŅµÉĻ³£ÓĆĮņ»Æ¼ī·ØÖʱøNa2S2O3£¬·“Ó¦ŌĄķĪŖ£ŗ
2Na2S+Na2CO3+4SO2 3Na2S2O3+CO2£¬Ä³ŃŠ¾æŠ”×éŌŚŹµŃéŹŅÄ£ÄāøĆ¹¤ŅµŌĄķÖʱøNa2S2O3£¬²æ·ÖŹµŃé×°ÖĆČēĻĀ:
3Na2S2O3+CO2£¬Ä³ŃŠ¾æŠ”×éŌŚŹµŃéŹŅÄ£ÄāøĆ¹¤ŅµŌĄķÖʱøNa2S2O3£¬²æ·ÖŹµŃé×°ÖĆČēĻĀ:
¢Ł×°ÖĆBµÄ×÷ÓĆŹĒ¼ģŃé×°ÖĆAÖŠSO2µÄĪüŹÕŠ§ĀŹ£¬ŌņBÖŠŹŌ¼ĮŹĒ________________£¬±ķĆ÷SO2ĪüŹÕŠ§ĀŹµĶµÄŹµŃéĻÖĻóŹĒBÖŠČÜŅŗ________________________”£
¢ŚŹµŃé½įŹųŹ±£¬²āµĆ×°ÖĆCÖŠµÄČÜŅŗŗ¬ÓŠĮ½ÖÖČÜÖŹ£¬ĘäÖŠŅ»ÖÖĪŖNaOH£¬ŌņŹµŃé¹ż³ĢÖŠøĆ×°ÖĆÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_________________________£»ČōĮ½ÖÖČÜÖŹµÄĪļÖŹµÄĮæĻąµČ£¬ŌņøĆČÜŅŗĖłŗ¬Ąė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖ__________________________________”£
¢Ū¼ŁÉč±¾ŹµŃéĖłÓƵÄNa2CO3ŗ¬ÉŁĮæNaC1”¢NaOH£¬Éč¼ĘŹµŃé·½°ø½ųŠŠ¼ģŃ锣ĒėĶź³ÉĻĀ±ķ”£
ŅŃÖŖ£ŗŹŅĪĀŹ±CaCO3±„ŗĶČÜŅŗµÄpH=10£®2”£
ĻŽŃ”ŹŌ¼Į¼°ŅĒĘ÷£ŗĻ”ĻõĖį”¢AgNO3ČÜŅŗ”¢CaC12ČÜŅŗ”¢·ÓĢŖČÜŅŗ”¢ÕōĮóĖ®”¢pH¼Ę£¬ÉÕ±”¢ŹŌ¹Ü”¢½ŗĶ·µĪ¹Ü”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø1£©Ä³Ń§ÉśÓūÓĆ11.9 mol”¤L-1µÄÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ500 mLĪļÖŹµÄĮæÅضČĪŖ0.400 mol”¤L-1µÄĻ”ŃĪĖį”£øĆѧɜŠčŅŖĮæČ”________mLÅØŃĪĖį½ųŠŠÅäÖĘ”££Ø±£ĮōŠ”Źżµćŗó1Ī»£©
£Ø2£©ČŻĮæĘæŹĒÅäÖĘČÜŅŗµÄ±ŲŠčŅĒĘ÷”£ĻĀĮŠ¹ŲÓŚČŻĮæĘæ¼°ĘäŹ¹ÓĆ·½·ØµÄŠšŹö£¬“ķĪóµÄŹĒ
¢ŁŹĒÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗµÄ×ØÓĆŅĒĘ÷
¢ŚŹ¹ÓĆĒ°ŅŖĻČ¼ģ²éČŻĮæĘæŹĒ·ńĀ©Ņŗ
¢ŪČŻĮæĘææÉŅŌÓĆĄ“¼ÓČČ
¢Ü²»ÄÜÓĆČŻĮæĘæ³¤ĘŚÖü“ęÅäÖĘŗƵÄČÜŅŗ
¢ŻæÉŅŌÓĆ500mLČŻĮæĘæÅäÖĘ250mLČÜŅŗ
¢ŽČŻĮæĘæÉĻ±źÓŠµÄŹĒĪĀ¶Č ”¢ČŻĮæ ”¢æĢ¶ČĻß
a£®¢Ł ¢Ū b£®¢Ł ¢Ü c£®¢Ū ¢Ż d£®¢Ż ¢Ž
£Ø3£©¢Ł øĆĶ¬Ń§ÓĆÅäÖʵÄ0.400 mol”¤L-1µÄŃĪĖį£¬ÖŠŗĶŗ¬0.4 g NaOHµÄNaOHČÜŅŗ£¬ŌņøĆĶ¬Ń§ŠčČ”________mLŃĪĖį”£
¢Ś ¼ŁÉčøĆĶ¬Ń§ÓĆŠĀÅäÖʵÄŃĪĖįÖŠŗĶŗ¬0.4 g NaOHµÄNaOHČÜŅŗ£¬·¢ĻÖ±Č¢ŁÖŠĖłĒóĢå»żĘ«Š”£¬ŌņæÉÄܵÄŌŅņŹĒ________”£
a£®ÅØŃĪĖį»Ó·¢£¬ÅØ¶Č²»×ć
b£®ÅäÖĘČÜŅŗŹ±£¬Ī“Ļ“µÓÉÕ±
c£®ÅäÖĘČÜŅŗŹ±£¬ø©ŹÓČŻĮæĘææĢ¶ČĻß
d£®¼ÓĖ®Ź±³¬¹żæĢ¶ČĻߣ¬ÓĆ½ŗĶ·µĪ¹ÜĪü³ö
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŗ£Ė®ÖŠŌĢ²Ų×Å·įø»µÄ׏Ō“”£ŗ£Ė®×ŪŗĻĄūÓƵÄĮ÷³ĢĶ¼ČēĻĀ”£
£Ø1£©ÓĆNaCl×öŌĮĻæÉŅŌµĆµ½¶ąÖÖ²śĘ·”£
¢Ł ¹¤ŅµÉĻÓÉNaClÖʱø½šŹōÄʵĻÆѧ·½³ĢŹ½ŹĒ_______________________________”£
¢Śµē½āĀČ»ÆÄĘĻ”ČÜŅŗæÉÖʱø”°84Ļū¶¾Ņŗ”±£¬ĶصēŹ±ĀČĘų±»ČÜŅŗĶźČ«ĪüŹÕ£¬ČōĖłµĆĻū¶¾Ņŗ½öŗ¬Ņ»ÖÖČÜÖŹ£¬Š“³öĻąÓ¦µÄ»Æѧ·½³ĢŹ½£ŗ____________________________”£
£Ø2£©·ÖĄė³ö“ÖŃĪŗóµÄĀ±Ė®ÖŠŌĢŗ¬×Å·įø»µÄĆ¾×ŹŌ“£¬¾¹żĻĀĮŠĶ¾¾¶æÉ»ńµĆ½šŹōĆ¾£ŗ
Ā±Ė® Mg£ØOH£©2
Mg£ØOH£©2 MgCl2ČÜŅŗ”śMgCl2”¤6H2O”śMgCl2
MgCl2ČÜŅŗ”śMgCl2”¤6H2O”śMgCl2 Mg
Mg
ĘäÖŠ£¬ÓÉMgCl2”¤6H2OÖĘČ”ĪŽĖ®MgCl2µÄ²æ·Ö×°ÖĆ£ØĢś¼ÜĢØ”¢¾Ę¾«µĘŅŃĀŌ£©ČēĻĀ£ŗ
¢ŁÉĻĶ¼ÖŠ£¬×°ÖĆaÓÉ ”¢ ”¢Ė«æ×ČūŗĶµ¼¹Ü×é³É”£
¢ŚŃ»·ĪļÖŹ¼×µÄĆū³ĘŹĒ ”£
¢ŪÖĘČ”ĪŽĖ®ĀČ»ÆĆ¾±ŲŠėŌŚĀČ»ÆĒā“ęŌŚµÄĢõ¼žĻĀ½ųŠŠ£¬ŌŅņŹĒ ”£
¢Ü×°ÖĆbÖŠĢī³äµÄĪļÖŹæÉÄÜŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com