
|
ij��Ԫ��(H2A)����ʽ�������룺H2A��H+��HA����HA�� ��0.01mol/L��H2A��Һ��0.01mol/L��NaHA��Һ ��0.02mol/L��HCl��Һ��0.04mol/L��NaHA��Һ�������� ��0.02mol/L��NaOH��Һ��0.02mol/L��NaHA��Һ�������� | |
| [����] | |
A�� |
������Һ��c(HA��)Ũ�ȴ�С���ۣ��٣��ڣ��� |
B�� |
��Һ��������Ũ�ȴ�С˳���ǣ�c(H2A)��c(H��)��c(HA��)��c(A2��)��c(OH��) |
C�� |
��Һ�����й�����Ũ�ȹ�ϵ��c(HA��)��2c(A2��)��c(H2A)��c(Na+) |
D�� |
��Һ�����й�����Ũ�ȹ�ϵ��c(HA��)��c(A2��)��c(OH��)��c(Na+)��c(H��) |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�������ѧ2012�������һѧ�����п��Ի�ѧ���� ���ͣ�013
|
ij��Ԫ��(H2A)����ʽ�������룺H2A��H+��HA����HA�� ��0.01 mol/L��H2A��Һ ��0.01 mol/L��NaHA��Һ ��0.02 mol/L��HC1��Һ��0.04 mol/L��NaHA��Һ�������� ��0.02 mol/L��NaOH��Һ��0.02 mol/L��NaHA��Һ�������� ���й�������������Һ��˵����ȷ���� | |
| [����] | |
A�� |
��Һ ���д���ˮ��ƽ����HA����H2O |
B�� |
��Һ ��������c(HA��)��c(A2һ)��c(Na+) |
C�� |
��Һ ��������c(OHһ)��c(H+)��c(HA��)��2c(H2A) |
D�� |
������Һ�� c(HA��)Ũ�ȴ�С���ۣ��ڣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��н�ɽ��2011�����һģ��ѧ���� ���ͣ�021
|
ij��Ԫ��(H2A)����ʽ�������룺H2A��H+��HA����HA�� ��0.01 mol/L��H2A��Һ ��0.01 mol/L��NaHA��Һ ��0.02 mol/L��HCl��Һ��0.04 mol/L��NaHA��Һ�������� ��0.02 mol/L��NaOH��Һ��0.02 mol/L��NaHA��Һ�������� | |
A�� |
������Һ��c(HA��)Ũ�ȴ�С���ۣ��٣��ڣ��� |
B�� |
��Һ����һ��������OH�� |
C�� |
��Һ���д���ˮ��ƽ�⣺HA����H2O |
D�� |
��Һ�����й�����Ũ�ȹ�ϵ��c(HA��)��c(A2��)��c(Na+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡģ���� ���ͣ���ѡ��
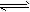 H++A2-����������������Һ����0.01 mol/L��
H++A2-����������������Һ����0.01 mol/L��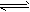 H2A+OH-
H2A+OH-�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com