

���� ��1�����ʵ��������A�к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�
��2���ٲⶨ����A��SO2�ĺ�����Ϊ�˱�֤ʵ��ɹ���Ҫ��Aװ�����ø��������Һȫ�����գ����������Һ����ɫ֤��������ȫ���������Ҫ�����dz�����ն���������������ʣ�
�ڲⶨ�Ľ��Ӧ��ƫ���������ɵ������ᱻ����������������������������
��3���������������������������������Ϊ���ᣬ����������ԭΪ�����������������п�ۻ�ԭ�������Ӻ������ӣ��õ�����AΪп��������ҺΪ����п��Һ��ͨ������Ũ������ȴ�ᾧ������ϴ�Ӹ���Ȳ���õ�����п���壻
�١��������м���������������Ӧ�Ǻ������ж��������������з�Ӧ�����������������
�ڼ������п�ۻ�ԭ�������Ӻ������ӣ�
�۷�����֪����AΪп������
��� �⣺��1�����Ṥҵ�����ķ���A����Ҫ�ɷ֣�SO2��O2��N2��CO2�ȣ��ŷŵ������л���Ⱦ���������ʵ��������A�к��е�CO2����Ҫ��ȥ������������Ŷ�����̼�ļ��飬�����������������ͨ������ʯ��ˮ���������̼�Ĵ��ڣ�ʯ��ˮ�����֤�����ж�����̼������A��Ϊ���������Һ��B��Ϊ����ʯ��ˮ��
�ʴ�Ϊ��BC��
��2����Ϊ�˱�֤ʵ��ɹ���Ҫ��Aװ�����ø��������Һȫ�����գ����������Һ����ɫ֤����������������ȫ���������Ҫ�����dz�����ն���������������ʣ�
�ʴ�Ϊ��KMnO4��Һ���Ϻ�ɫ������ȫ��ɫ�����SO2�������ʣ�
�ڰ���ͬѧ����ʵ�飬��������SO2��ȫ�����գ��ⶨ�Ľ��Ӧ��ƫ���������ɵ������ᱻ����������������ʵ������з���2H2SO3+O2=2H2SO4�����²ⶨ������������
�ʴ�Ϊ��ʵ������з�����2H2SO3+O2=2H2SO4��
��3���������������������������������Ϊ���ᣬ����������ԭΪ�����������������п�ۻ�ԭ�������Ӻ������ӣ��õ�����AΪп��������ҺΪ����п��Һ��ͨ������Ũ������ȴ�ᾧ������ϴ�Ӹ���Ȳ���õ�����п���壬
�١��������м���������������Ӧ�Ǻ������ж��������������з�Ӧ�����������������ᣬ��Ӧ�����ӷ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
�ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
�ڼ������п�ۻ�ԭ�������Ӻ������ӣ�����Һ�е�Fe2+��Fe3+�ȣ�
�ʴ�Ϊ������Һ�е�Fe2+��Fe3+�ȣ�
�۷�����֪����AΪп������
�ʴ�Ϊ��Zn��Fe��
���� ���⿼����������ɵ�ʵ��̽���������̷����жϣ���Ҫ���������ʵ�����Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ | B�� | ϡ���� | C�� | ����������Һ | D�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol�Ȼ�������ˮ���1 L��Һ��������Һ��Cl-�����ʵ���Ũ��Ϊ1mol/L | |
| B�� | �ڱ�״���£�11.2LH2O��������ԭ����ΪNA | |
| C�� | CO2��Ħ������Ϊ44g | |
| D�� | ��״����22.4LCH4��l8gH2O�����еĵ�������Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��H+��=10-12 mol/L����Һ��K+��Ba2+��Cl-��Br- | |
| B�� | ʹ��ɫʯ����Һ������Һ��Fe2+��Mg2+��NO3-��Cl- | |
| C�� | ̼��������Һ��K+��SO42-��Cl-��H+ | |
| D�� | ʹ��̪��Һ������Һ��Na+��Cl-��SO42-��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1����������������
��1���������������� ��
�� ������ ��C��CH3��4��
������ ��C��CH3��4��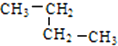 �����л�Ϊͬ���칹����Ǣڢܣ�����ţ��������
�����л�Ϊͬ���칹����Ǣڢܣ�����ţ�������� ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������
����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������| A�� | �������������������½��ȶ� | |
| B�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ֵKa=10-6 | |
| C�� | pHԽ��Ư����Ư������Խ�� | |
| D�� | 25�棬pH=3ʱ��NaClO2��Һ�У�c��Na+��+c��H+��=c��ClO2-��+c��OH-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com