


 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
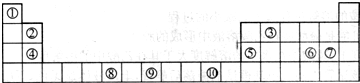
| �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | ||||||||||||||||
| �� | �� |

| M | ||
4
|
| M | ||
4
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣��±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ��
�����
��1��д���١�����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ĵ���ʽ ��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ ��
��2��Ԫ�آ�����γɵĻ�����ľ��������ǣ�_ _ _��
��3��Ԫ�آݡ��ĵ�һ�����ܴ�С˳���ǣ� �� ����Ԫ�ط��ű�ʾ����Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ�� ����д��һ����N3����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ
��4���ߡ�������Ԫ�����γ�һ��AB2�͵Ĺ��۷��ӣ��÷������� ���ӣ�����ԡ��Ǽ��ԡ����ݡ��ߡ� ������Ԫ��֮������ԭ�Ӹ�����1��1�����γɻ������Щ�������������������ЩԪ�صĵ��ʡ���д���ߡ�������Ԫ���γɵĻ�����Ļ�ѧʽ ������Ԫ��д��ǰ�棩��
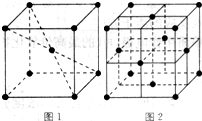
��5��Ԫ�آ���Ԫ�����ڱ����������� ��Ԫ�أ�Ԫ�آ���һ���������γɵľ�������־�������ͼ1��ͼ2��ʾ������ͼ1��ͼ2�Ľṹ�����Ԫ��һ��ԭ�ӵȾ����������ԭ����֮��Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��2010Ȫ������ѧ�����ѧ�ڸ߶������п��Ի�ѧ�� ���ͣ������
��16�֣��±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ��

�����
��1��д���١�����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ĵ���ʽ ��д���ϱ���Ԫ�آ�ԭ�ӵ���Χ�����Ų�ʽ ��
��2��Ԫ�آ�����γɵĻ�����ľ��������ǣ�_ _ _��
��3��Ԫ�آݡ��ĵ�һ�����ܴ�С˳���ǣ� �� ����Ԫ�ط��ű�ʾ����Ԫ�آ���Ԫ�آ��γɵ�X���ӵĿռ乹��Ϊ�� ����д��һ����N3����Ϊ�ȵ�����ķ��ӵĻ�ѧʽ 
��4���ߡ�������Ԫ�����γ�һ��AB2�͵Ĺ��۷��ӣ��÷������� ���ӣ�����ԡ��Ǽ��ԡ����ݡ��ߡ�������Ԫ��֮������ԭ�Ӹ�����1��1�����γɻ������Щ�������������������ЩԪ�صĵ��ʡ���д���ߡ�������Ԫ���γɵĻ�����Ļ�ѧʽ ������Ԫ��д��ǰ�棩��
��5��Ԫ�آ���Ԫ�����ڱ����������� ��Ԫ�أ�Ԫ�آ���һ���������γɵľ�������־�������ͼ1��ͼ2��ʾ������ͼ1��ͼ2�Ľṹ�����Ԫ��һ��ԭ�ӵȾ����������ԭ����֮��Ϊ�� ��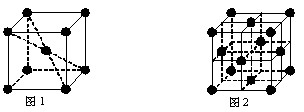
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ����һ�и���9�³���⻯ѧ�Ծ� ���ͣ������
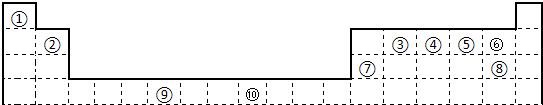
��12�֣��±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�����ա�
| �� | | | | | | | | | | | | | | | | | |
| | �� | | | | | | | | | | | | �� | �� | �� | �� | |
| | | | | | | | | | | | | �� | | | | �� | |
| | | | | | �� | | | �� | | | | | | | | | |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com