
2013�����ȫ�����ض�����ж�������ʮ������������ɡ������족����Ҫ��Դ֮һ������β����ȼúβ���ŷų����Ĺ���С������
����β����������Ҫԭ��Ϊ��2NO(g)+2CO(g) 2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ�
2CO2+N2�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯��������ͼ��ʾ���ݴ��жϣ� 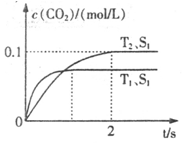
��1���÷�ӦΪ ��Ӧ(����ȡ������ȡ�������T2�¶��£�0~2s�ڵ�ƽ����Ӧ���ʣ�v(N2)= ����2�����������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2���ڴ���ϻ��� c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
��3��ij���л�������t1���£�����㶨���ܱ������У������崫��������˲�ͬʱ���NO��CO��Ũ�ȣ��������ݼ��±���CO2��N2����ʼŨ��Ϊ0����
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c(NO)/xl0-4mol L-1 | 10��0 | 4��50 | 2��50 | 1��50 | 1��00 | 1��00 |
| c(CO)/xl0-3mol L-1 | 3��60 | 3��05 | 2��85 | 2��75 | 2��70 | 2��70 |
 N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1
��1������ 0��025 mol/(L��s) ����2�֣���4�֣�
��2����ͼ����T1S1�·�����㲻�䡢�յ������ߺɣ��������ɣ���2�֣�

��3��5000 L/mol ��2�֣� 2��41% (2��)
��4��B D ����2�֣���ѡ��1�֣���ѡ���÷֣�
��5��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol ��2�֣�
������������������ݡ��ȹ���ƽ����ֵ��ԭ��֪��T1��T2������T1�����¿�֪��������̼�ĺ��������ͣ���֪�������¶ȷ�Ӧ�����ƶ�����������Ƿ��ȵġ���ѧ��Ӧ���ʵ��ڵ�λʱ���ڷ�Ӧ�����������Ũ�ȵı仯������˿���ȷ���ö�����̼��ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.050 mol/(L��s)�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���֪���õ�����ʾ�Ļ�ѧ��Ӧ���ʾ�Ϊ��0.025 mol/(L��s)��������������ֻ������Ӧ���ʣ�����Ӱ�������̼�ĺ�������ˣ�ֻ�Ƿ�Ӧ�ﵽƽ���ʱ���ӳ�������Ӱ�������̼������
�� 2NO(g) + 2CO(g)  2CO2 + N2
2CO2 + N2
��ʼŨ�ȣ�10��3mol/L 3.6��10��3mol/L 0 0
ת��Ũ�ȣ�9.0��10��4mol/L 9.0��10��4mol/L 9.0��10��4mol/L 4.5��10��4mol/L
ƽ��Ũ�ȣ�10��4mol/L 2.7��10��3mol/L 9.0��10��4mol/L 4.5��10��4mol/L
���ƽ�ⳣ����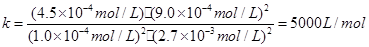
��駣�ƽ��ʱ����ȵ������ʵ���֮�ȣ�����Ũ��֮�ȣ�����ƽ��ʱNO���������Ϊ��

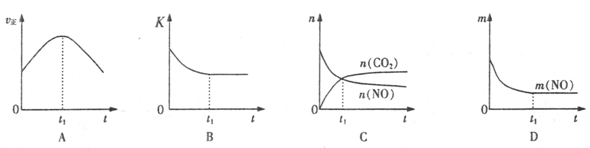
��Aͼ������Ӧ���ʷ�Ӧ��ʼ���Ǽ�С�ģ�����Bͼ�б�ʾ���ǻ�ѧƽ�ⳣ����ʱ��ı仯����Ϊ�Ǿ��ȵģ�����Ӧ���Ƿ��ȵģ��������ŷ�Ӧ�Ľ��У��ų�������ɢ����ȥ��ʹ��Ӧ�����ƶ���ʹ��ƽ�ⳣ����С������ijһ��ʱ�̴ﵽƽ��״̬����ȷ��Cͼ����t1����Ե����ʵ������ڱ仯������ƽ��״̬������Dͼ��һ��������������t1ʱ���������䣬һ����һ��ƽ��״̬����ȷ��
�� ��CH4(g)+2NO2(g) = N2 (g)+CO2 (g)+2H2O(g) ��H=-867��0kJ ? mol-1
��2NO2 (g)  N2O4 (g) ��H=-56��9kJ ? mol-1
N2O4 (g) ��H=-56��9kJ ? mol-1
��H2O(g) = H2O(l) ��H=-44��0kJ ? mol-1
�٣��ڣ��ۡ�2��CH4(g)+N2O4(g)�TN2(g)+CO2(g)+2H2O(g) D H�T ��898��1kJ/mol
���㣺���黯ѧƽ�⣬�Ȼ�ѧ���й�֪ʶ��


 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(16��) ����P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���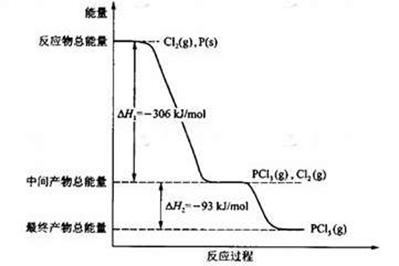
������ͼ�ش��������⣺��1��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ��
��
��2��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ��
��
�����ֽⷴӦ��һ�����淴Ӧ���¶�T1ʱ�����ܱ������м���0.80mol PCl5����Ӧ��ƽ��ʱPCl5��ʣ0.60 mol����ֽ���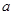 1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ
1���� ������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ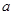 2��
2��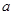 2
2 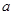 1������ڡ�����С�ڡ����ڡ�����
1������ڡ�����С�ڡ����ڡ�����
��3����ҵ���Ʊ�PCl5ͨ�����������У��ֽ�P��Cl2��Ӧ�����м����PCl3��Ȼ���£��ٺ�Cl2��Ӧ����PCl5��ԭ����
��
��4��P��Cl2��������Ӧ����1 mol PCl5�ġ�H 3= ��P��Cl2һ����Ӧ����1 mol PCl5�ġ�H 4 ��H 3������ڡ�����С�ڡ����ڡ�����
��5��PCl5������ˮ��ַ�Ӧ���������������ᣬ�仯ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��
CH4(g)��CO2(g)=2CO(g)��2H2(g)��H����260 kJ/mol
��֪��2CO(g)��O2(g)=2CO2(g) ��H����566 kJ/mol
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ�ֿ�����������ȼ�ϣ���;�㷺���о������þ��й���ǰ����
��1����֪�ڳ��³�ѹ�£���÷�Ӧ�ķ�Ӧ�����£�
�� 2CH3OH(l)+ 3O2(g)  2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
�� 2CO(g) +O2(g)  2CO2(g) ?H2=��566��0 kJ/mol
2CO2(g) ?H2=��566��0 kJ/mol
CH3OH����ȫȼ������CO����̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�������״��ķ�Ӧ���£�CO2(g) + 3H2(g)  CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
��ij�¶��£��ݻ���Ϊ1 L��A��B���������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݡ�����B�о�10 s��ﵽƽ�⡣�ﵽƽ��ʱ���й��������±���
| ���� | A | B |
| ��Ӧ��Ͷ���� | 1 mol CO2(g)��3 mol H2(g) | 1 mol CH3OH(g)��1 mol H2O(g) |
| ��Ӧ�����仯 | �ų���kJ���� | ����19��6 kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H����260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g) ��H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_______________________________
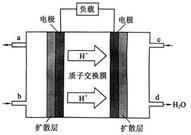
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��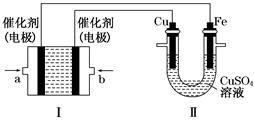
��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��______________________________
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I�� C(s)��O2(g) = CO2(g)
;��II������ˮú���� C(s) + H2O(g) =" CO(g)" + H2(g)
ȼ��ˮú����2 CO(g) + O2(g) = 2CO2(g)��
2H2(g)+O2(g) =2H2O(g)
��֪����C(s)��O2(g)=CO2(g)����H1=��393.5 kJ��mol-1
��H2(g)+1/2O2(g)=H2O(g)����H2=��241.8kJ��mol-1
��CO(g)+ 1/2O2 (g) =CO2(g)����H3=��283.0kJ��mol-1
��ش��������⣺
��1��CO(g) + H2O(g) = H2(g) + CO2(g) �� ������ȷ�Ӧ�������ȷ�Ӧ����
��2�����ݸ�˹���ɣ�ú����̬ˮ����ˮú���ķ�Ӧ�ȡ�H= ��
��3����������;��������˵��������ǣ� ��
| A��;��II��ˮú��ʱ�����ܺģ���;��II����������ȡ |
| B����;��I��ȣ�;��II���Լ��ٶԻ�������Ⱦ |
| C����;��I��ȣ�;��II�������ú��ȼ��Ч�� |
| D����úת��Ϊˮú������ͨ���ܵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ú��Ϊȼ�Ͽ�ͨ����������;��:
;����C(s)+O2(g) CO2(g)����H1<0��
CO2(g)����H1<0��
;�������Ƴ�ˮú��:
C(s)+H2O(g) CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0��
��ȼ��ˮú��:
2CO(g)+O2(g) 2CO2(g)����H3<0��
2CO2(g)����H3<0��
2H2(g)+O2(g) 2H2O(g)����H4<0��
2H2O(g)����H4<0��
��ش���������:
(1);����ų���������������������(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ������
(3)��֪:��C(s)+O2(g) CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1
��2CO(g)+O2(g) 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1
��TiO2(s)+2Cl2(g) TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s) TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=����������
(4)��֪���и����Ȼ�ѧ����ʽ
��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
��3Fe2O3(s)+CO(g) 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
��Fe3O4(s)+CO(g) 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
��д��FeO(s)��CO(g)��ԭ��Fe��CO2(g)���Ȼ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��úת��Ϊˮú����ͨ����ѧ������úת��Ϊ�ྻȼ�ϵķ���֮һ��úת��Ϊˮú������Ҫ��ѧ��ӦΪ��C(s)��H2O(g) CO(g)��H2(g)��
CO(g)��H2(g)��
��C(g)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
��C(s)��O2(g)=CO2(g)����H1����393.5 kJ��mol��1
��H2(g)�� O2(g)=H2O(g)����H2����242.0 kJ��mol��1
O2(g)=H2O(g)����H2����242.0 kJ��mol��1
��CO(g)�� O2(g)=CO2(g)����H3����283.0 kJ��mol��1
O2(g)=CO2(g)����H3����283.0 kJ��mol��1
�������������Ϣ�ش��������⣺
(1)ú��һ�ֳɷָ��ӵĻ������г���̼����Ԫ���⣬���������������顢����Ԫ�ء����Թ���úȼ�ջᵼ�´�����Ⱦ��д��úȼ�ղ�����������Ⱦ����������������������ʯ��ʯ����ú�ۻ�ϣ�������Ч�ؼ���úȼ�չ����еĶ���������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________
(2)������֪�Ȼ�ѧ����ʽд����ú�Ʊ�ˮú�����Ȼ�ѧ����ʽ��____________________________��
(3)�����Ǽס�����λͬѧ�������Ȼ�ѧ����ʽ��úȼ�յ����⡣
��ͬѧ��1 mol CO��1 mol H2ȼ�շų�������֮�ʹ���1 mol����̿ȼ�շų�������������úȼ��ʱ��������ˮ������ʹúȼ�շų������������
��ͬѧ���������������������ѭ������ú̿ת��Ϊˮú������ȼ�շų���������ֱ��ȼ��ú̿�ų���������ͬ������ú̿ת��Ϊˮú�������������ģ���ú̿ת��Ϊˮú���ò���ʧ��
C(s)��H2O(g)��O2(g) CO2(g)��H2O(g)
CO2(g)��H2O(g)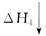

CO(g)��O2(g)��H2(g)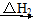 CO(g)��H2O(g)��
CO(g)��H2O(g)�� O2(g)
O2(g)
����������λͬѧ�����⣺
�ټ�ͬѧ��˵������������(����ȷ������ȷ��)��ԭ����______________________________________��
����ͬѧ��˵������������(����ȷ������ȷ��)��ԭ����_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӡ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ��mol��1��ʾ��������۲���ͼ��Ȼ��ش����⡣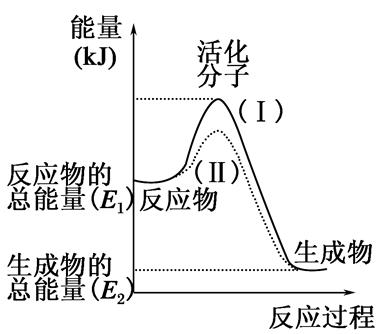
��1��ͼ����ʾ��Ӧ��________������ȡ����ȡ�����Ӧ��
��2����֪��1mol H��H����1mol I��I��1mol H��I���ֱ���Ҫ���յ�����Ϊ436kJ��151kJ��299kJ������1mol������1mol �ⷴӦ����HI��________����ų��������ա���________kJ���������ڻ�ѧ��Ӧ�����У��ǽ�______ת��Ϊ________��
��3�����з�Ӧ�У����ڷ��ȷ�Ӧ����________���������ȷ�Ӧ����________��
������ȼ��
��ըҩ��ը
������кͷ�Ӧ
�ܶ�����̼ͨ�����ȵ�̼
��ʳ��������������
��Ba��OH��2��8H2O��NH4Cl��Ӧ
��������ϡ���ᷴӦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com