
ТСЦЄЈєўЩNaNO2ѕЯУРЗїСх»ЇРФ
ўЪ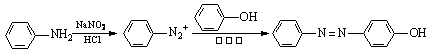
°ВЙіАЗШКЗТ»ЦЦї№ѕъТ©ЎЈЖдєПіЙВ·ПЯИзПВЈє
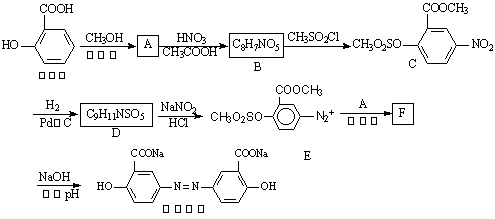
ЈЁ1Ј©РґіцПВБР·ґУ¦µД»ЇС§·ЅіМКЅЈє
Л®СоЛбЎъA_________________________________________________________Ј»
AЎъB__________________________________________________Ј»
ЈЁ2Ј©РґіцDµДЅб№№јтКЅ______________________Ј»
ЈЁ3Ј©РґіцПВБР·ґУ¦µД·ґУ¦АаРНЈєDЎъE______________Ј¬FЎъ°ВЙіАЗШ___________Ј»
ЈЁ4Ј©ґУХыёцєПіЙВ·ПЯїґЈ¬ЙијЖBЎъCІЅЦиµДЧчУГКЗ___________________________Ј»
ЈЁ5Ј©FµДЅб№№јтКЅОЄ___________________Ј»
 ЈЁ6Ј©УР»ъОпGЈЁЅб№№јтКЅјыПВНјЈ©ТІїЙУГУЪєПіЙ°ВЙіАЗШЎЈЛьµДТ»ЦЦН¬·ЦТм№№МеXКЗ
ЈЁ6Ј©УР»ъОпGЈЁЅб№№јтКЅјыПВНјЈ©ТІїЙУГУЪєПіЙ°ВЙіАЗШЎЈЛьµДТ»ЦЦН¬·ЦТм№№МеXКЗ![]() Ј°±»щЛбЈ¬ДЬУлFeCl3·ўЙъПФЙ«·ґУ¦Ј¬Жд·ЦЧУЦР№ІУР6ЦЦ»ЇС§»·ѕіІ»Н¬µДHФЧУЎЈXµДЅб№№јтКЅОЄ________________
Ј°±»щЛбЈ¬ДЬУлFeCl3·ўЙъПФЙ«·ґУ¦Ј¬Жд·ЦЧУЦР№ІУР6ЦЦ»ЇС§»·ѕіІ»Н¬µДHФЧУЎЈXµДЅб№№јтКЅОЄ________________
 ЕаУЕїЪЛгМвїЁПµБРґр°ё
ЕаУЕїЪЛгМвїЁПµБРґр°ё їЄРДїЪЛгМвїЁПµБРґр°ё
їЄРДїЪЛгМвїЁПµБРґр°ё їЪЛгМвїЁєУ±±ЙЩДк¶щНЇіц°жЙзПµБРґр°ё
їЪЛгМвїЁєУ±±ЙЩДк¶щНЇіц°жЙзПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈєДЈДвМв МвРНЈєМоїХМв
 N2O4(g)µДЖЅєвіЈКэK=___Ј¬ИфNO2µДЖрКјЕЁ¶ИОЄ2 mol/LЈ¬ЗТіхКјК±ОЮN2O4Ј¬ЖдЛыМхјю±ЈіЦІ»±дЈ¬ФтNO2µДЧоґуЧЄ»ЇВКОЄ___ЎЈ
N2O4(g)µДЖЅєвіЈКэK=___Ј¬ИфNO2µДЖрКјЕЁ¶ИОЄ2 mol/LЈ¬ЗТіхКјК±ОЮN2O4Ј¬ЖдЛыМхјю±ЈіЦІ»±дЈ¬ФтNO2µДЧоґуЧЄ»ЇВКОЄ___ЎЈ Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com