
���� ��1������n=$\frac{m}{M}$�����������ʵ�����ԭ�����ʵ���Ϊ�������2�����ٸ���N=nNA����ԭ����Ŀ��
�ڸ���c=$\frac{n}{V}$�����������Һ��Ũ�ȣ�
��2��һ���¶���ѹǿ�£���������֮�ȵ������ʵ���֮�ȣ����ڻ�ѧ������֮�ȣ�Ȼ�����������غ㶨����ȷ����ѧʽ��
��3����Һ�д��ڵ���غ㣬���ݵ���غ�c��Na+��+2c��Mg2+��=c��Cl-��+2c��SO42-���������Һ����������ӵ����ʵ���Ũ�ȣ�
��� �⣺��1����mg����������ʵ���Ϊ��$\frac{mg}{Mg/mol}$=$\frac{m}{M}$mol��������Ϊ˫ԭ�ӷ��ӣ����е�ԭ����Ϊ��$\frac{m}{M}$mol��2��NA=$\frac{{2m{N_A}}}{M}$mol��
�ʴ�Ϊ��$\frac{{2m{N_A}}}{M}$��
��������Һ�����ʵ���Ũ��Ϊ��$\frac{\frac{m}{M}mol}{VL}$=$\frac{m}{MV}$mol/L��
�ʴ�Ϊ��$\frac{m}{MV}$mol/L��
��2��һ���¶Ⱥ�ѹǿ�£�1���X2������3���Y2���廯������2������廯�����û�����ΪZ������������֮�ȵ������ʵ���֮�ȣ����ڻ�ѧ������֮�ȣ�
��X2+3Y2�T2Z����ԭ���غ��֪��ZΪXY3��Y3X��
�ʴ�Ϊ��XY3��Y3X��
��3�����ݵ���غ�ɵã�c��Na+��+2c��Mg2+��=c��Cl-��+2c��SO42-����
��0.2mol•L-1+2��0.25mol•L-1=0.4mol•L-1+2c��SO42-����
��ã�c��SO42-��=0.15 mol•L-1
�ʴ�Ϊ��0.15 mol•L-1��
���� ���⿼�����ʵ���������٤�����ɼ��������йؼ��㣬��Ŀ�Ѷ��еȣ���ȷһ���¶���ѹǿ����������뻯ѧ�������Ĺ�ϵ����Ϥ�������㹫ʽ���ɽ������������ѧ���Ļ�ѧ����������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| NH3•H2O | H2SO3 | ||
| ����ƽ�ⳣ����mol/L�� | 1.7��10-5 | Ka1 | Ka2 |
| 1.54��10-2 | 1.02��10-7 | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO32- | B�� | OF2 | C�� | GeBr2 | D�� | CCl4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
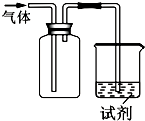 ��ͼװ�ÿ������ռ����岢��֤�仯ѧ���ʣ����ж�Ӧ��ϵ��ȫ��ȷ���ǣ�������
��ͼװ�ÿ������ռ����岢��֤�仯ѧ���ʣ����ж�Ӧ��ϵ��ȫ��ȷ���ǣ�������| ѡ�� | ���� | �Լ� | ���� | ���� |
| A | NO | ��ɫʯ����Һ | ��Һ��� | NO��ˮ��Ӧ�������� |
| B | Cl2 | KI������Һ | ��Һ���� | Cl2�������� |
| C | SO2 | ����KMnO4��Һ | ��Һ��ɫ | SO2��Ư���� |
| D | NH3 | ��̪�Լ� | ��Һ��� | NH3�м��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Na�Ż𣬿�������ˮ���� | |
| B�� | ����ʱ�����Ż��ˣ��ɹص���Դ���ù��Ǹ��� | |
| C�� | ���ȹ�����й©��Ӧ�����ȹ���Χ��������ϡNaOH��Һ | |
| D�� | ����������ζʱ��Ӧ������ƿ������ɿ����ʹ������������ǿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ����I | ����II |
| A | ʵ���ҳ���Al2��SO4��3��Һ�백ˮ��Һ�Ʊ�Al��OH��3���� | Al��OH��3�����ڼ� |
| B | NaHCO3Ϊǿ�������� | NaHCO3��Һ�Լ��� |
| C | SO2���������� | SO2����Ʒ����Һ���� |
| D | ����ˮ��������������ԵĽ��� | ������������ˮ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeO | B�� | Fe3O4 | C�� | Fe2O3 | D�� | Fe��OH��3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com