
ʵ�����ø��������Cl2�����ڵ�Sn��Ӧ��SnCl4��Sn+2Cl2![]() SnCl4ͬʱ�Ŵ������ȣ���֪SnCl4����������ɫҺ�壬�е㣺114�棬����ʪ�����㷢��ˮ�ⷴӦ(SnCl4+2H2O�T�TSnO2+4HCl)������ɫ������Sn���۵㣺231�棬��ͼ����ȡSnCl4��ʵ��װ�á��Իش��������⣺
SnCl4ͬʱ�Ŵ������ȣ���֪SnCl4����������ɫҺ�壬�е㣺114�棬����ʪ�����㷢��ˮ�ⷴӦ(SnCl4+2H2O�T�TSnO2+4HCl)������ɫ������Sn���۵㣺231�棬��ͼ����ȡSnCl4��ʵ��װ�á��Իش��������⣺

��1��װ��A�еķ�Ӧԭ��������Ϊ��MnO2��ŨH2SO4��ʳ�η�Ӧ������HCl������Cl2����������������NaHSO4��MnSO4��װ��A�з�Ӧ�Ļ�ѧ����ʽ______________________
________________________________��
��2��Ϊʹʵ��ɹ���A��B����Ҫ���ʵ���װ�ã��뽫�����ڿ��ڣ���ע������ʢ�ŵ�ҩƷ��
��3������ʵ��ʱ��Ӧ�ȵ�ȼ����д��ĸ����ͬ��________���ľƾ��ƣ�����Ӧ����SnCl4ʱ��ӦϨ��________���ľƾ��ƣ�������________________________��
��4��װ��C��������________________________________��
��5�����д�ʵ�飬Dװ�ú�Ӧ�����ӵ�װ�ü������ǣ��������ҵ�˳��д���������Ƽ����е�ҩƷ��___________________________________________________________________
___________________________________________________________________________________________________________________________________________________________________________________________________________________________________________��
��1��2NaCl+3H2SO4+MnO2 ��2��
��3��B B ������Ӧ�ų�����ά��Sn���ۻ� ��4��ʹSnCl4�������� ��5�����Ӹ���ܣ�װ�м�ʯ�ң���ֹˮ����������ƿʹSnCl4����ˮ�⡣����Cl2��β������װ�ã�װ��NaOH��Һ������Cl2�������������������Ӹ���ܣ�װ�м�ʯ�ң���ֹ������ˮ����������ƿ��ʹSnCl4����ˮ�⣻����Cl2��������������
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
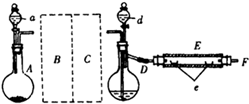 ��1����μ��Dװ�õ������ԣ�
��1����μ��Dװ�õ������ԣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
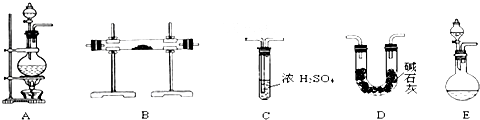
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
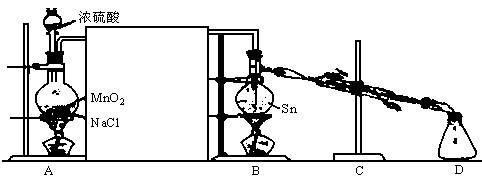
��1��װ��A�еķ�Ӧԭ��������Ϊ��MnO2��ŨH2SO4��ʳ�η�Ӧ������HCl������Cl2����������������NaHSO4��MnSO4��װ��A�з�Ӧ�Ļ�ѧ����ʽ______________________
________________________________��
��2��Ϊʹʵ��ɹ���A��B����Ҫ���ʵ���װ�ã��뽫�����ڿ��ڣ���ע������ʢ�ŵ�ҩƷ��
��3������ʵ��ʱ��Ӧ�ȵ�ȼ����д��ĸ����ͬ��________���ľƾ��ƣ�����Ӧ����SnCl4ʱ��ӦϨ��________���ľƾ��ƣ�������________________________��
��4��װ��C��������________________________________��
��5�����д�ʵ�飬Dװ�ú�Ӧ�����ӵ�װ�ü������ǣ��������ҵ�˳��д���������Ƽ����е�ҩƷ��___________________________________________________________________
___________________________________________________________________________________________________________________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��У��̳2011�������һ�����Ͽ��Ի�ѧ���� ���ͣ�058
SnCl4�ڴ���Diels��aider��Ӧ���Ӽ�������ֱ������ҪӦ�ã�ij��ѧ�о�С����̽��SnCl4���Ʊ���
[��������]
SnCl4�ڳ���������ɫҺ�壬�е�Ϊ114�棬����ʪ�����㷢��ˮ�ⷴӦ��Sn���۵�Ϊ231�森
[ʵ�鲽��]
��һ��������Sn���Ʊ���������ʯSnO2Ϊԭ�ϣ��ù����Ľ�̿����ԭ�����ڸ����¿��Ƶô�����
�ڶ�����SnCl4���Ʊ������ø��������C12�����ڵ�Sn��Ӧ�Ʊ�SnCl4ͬʱ�ų��������ȣ�
��ͼ��ʵ�����Ʊ�SnCl4��ʵ��װ�ã�
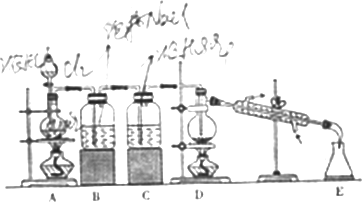
[����˼��]
(1)��һ����Ӧ�Ļ�ѧ����ʽΪ________��
(2)װ��B��C�е�ҩƷ�ֱ�Ϊ________��
(3)����Ӧ����SnCl4ʱ��ӦϨ��________���ľƾ��ƣ�������________��
[ʵ��Ľ�]
��ʦ˵װ��E��Ʋ�����������������������˵���ĵ�Ŀ�ģ�________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com