


 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

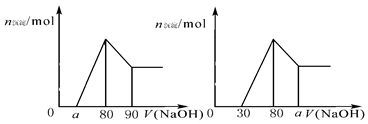
A��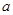 ��ȡֵ��ΧΪ0�� a��50 ��ȡֵ��ΧΪ0�� a��50 |
B��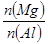 �����ֵΪ2.5 �����ֵΪ2.5 |
C��������ϵͼ��ΪBͼʱ����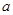 ��ȡֵ��ΧΪ80��a��90 ��ȡֵ��ΧΪ80��a��90 |
D��������ϵͼ��ΪCͼʱ����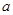 ��ȡֵ��ΧΪ75��a��90 ��ȡֵ��ΧΪ75��a��90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
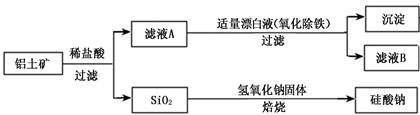
| A���Ʊ�AlCl3���ܲ��ý���Һֱ�����ɵķ��� |
| B����ҵ�ϲ��õ��AlCl3�ķ���ұ�������� |
| C��ʵ���ҳ��ð�ˮ����������Һ�Ʊ�Al(OH)3 |
| D����������ˮ�еĽ������ʣ�����Ͷ�������ȵ���ʵķ������д��� |
�鿴�𰸺ͽ���>>
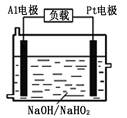
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
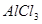 ��Һ�Ʊ�
��Һ�Ʊ�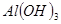 ���������ѡ�õ��Լ���
���������ѡ�õ��Լ���| A��ʯ��ˮ | B������������Һ | C������ | D����ˮ |
�鿴�𰸺ͽ���>>
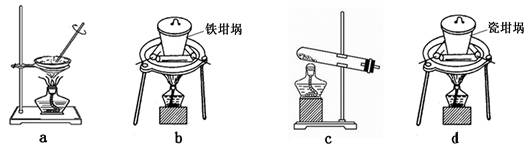
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
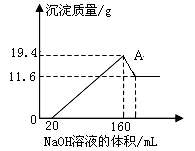
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Тڢ� | B��ֻ�Т٢� | C��ֻ�Т٢ڢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��72ml | B��80ml | C��90ml | D��120ml |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com