
+ 4 |
2- 3 |
2- 4 |
| ʵ����� | ʵ������ | ʵ���� |
| 1 | ����AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ��������BaCl2��Һ����Ӧ����С������������������м�����ϡ���ᡢȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������2.33g |
| 1.12L |
| 22.4L/mol |
| 2.33g |
| 233g/mol |
| 3.94g |
| 197g/mol |
| 0.02mol |
| 0.1L |
| 0.01mol |
| 0.1L |


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ��ʵ | ���� |
| A | ����ˮ��Ӧ��Na��Mg���� | �����ԣ�Na��Mg |
| B | Ca��OH��2�ļ���ǿ��Mg��OH��2 | �����ԣ�Ca��Mg |
| C | SO2��NaHCO3��Һ��Ӧ����CO2 | �ǽ����ԣ�S��C |
| D | t��ʱ��Br2+H2?2HBr K=5.6��107 I2+H2?2HI K=43 |
�ǽ����ԣ�Br��I |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
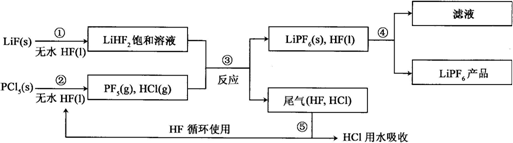
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
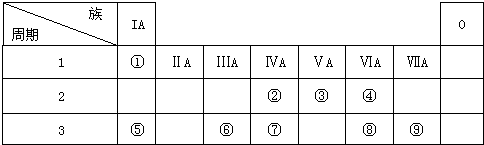
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
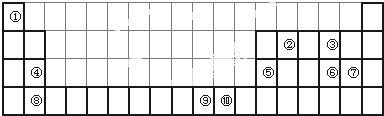
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Ʒ��ʼ����-a��������� |
| ��Ʒ��ʼ���� |
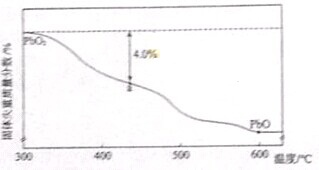
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| A����H2����H3 |
| B����H1����H3 |
| C����H1+��H3=��H2 |
| D����H1+��H2����H3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ�Ӱ뾶�Ĵ�С˳��r��W����r��Z����r��Y����r��X�� |
| B��Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ |
| C��X������������Ӧ��ˮ��������Ա�W���� |
| D��Y����̬���⻯������ȶ��Ա�W��ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڶ�����Ԫ���⻯���ȶ��Ե�˳���ǣ�HF��H2O�����Ե�������Ԫ���⻯���ȶ��Ե�˳��Ҳ�ǣ�HCl��H2S |
| B��IVA��Ԫ���⻯���۵�˳���ǣ�SiH4��CH4������VA��Ԫ���⻯���۵�˳��Ҳ�ǣ�PH3��NH3 |
| C����A��Ԫ�صķǽ������ǣ�F��Cl�����Ԣ�A��Ԫ���⻯�������Ҳ�ǣ�HF��HCl |
| D��þ�������ã���ҵ���õ�������������Ʊ��������Թ�ҵ��Ҳ�õ����������þ�Ʊ�þ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com