
����Ŀ������˵����ȷ����( )
A. ��ϵͳ�����������л���![]() ��
�� ������̼ԭ������Ϊ7��
������̼ԭ������Ϊ7��
B. ij���ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ���������������������4��
C. ̼ԭ����С�ڻ����8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ�����������ĵ�ϩ����6��
D. ���ⶨC3H7OH��C6H12��ɵĻ������������������Ϊ8%����˻������̼������������78%
���𰸡�D
�����������������A��![]() ��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ
��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ![]() �����Ի������C3H7OH��������Ϊ
�����Ի������C3H7OH��������Ϊ![]() =30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ
=30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ![]() ��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ
��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ![]() ��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��
��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ����ᡢ����������ء�����˵������ȷ���� ( )
A. �����ƿ��Ա�����ʯ�����У���������ͼ��־
B. ���������������ر���������Ϊ�����в�������������
C. ʯ�͵��ѽ���Եõ����顢��ϩ����ϩ����Ҫ����ԭ��
D. ú�к��б����ױ��ʹְ�ˮ����ͨ������õ�������Ʒ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ʣ�X�����л��ϳ�����Ҫ�м��壬���й�������˵����ȷ���ǣ� ��
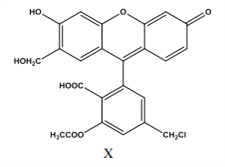
A. 1 mol X ���������� NaOH ��Һ��Ӧ�� ������� 6mol NaOH
B. 1 mol X ������� 10mol ������Ӧ������������������̼ԭ��
C. 1mol X ��������Ũ��ˮ��Ӧ��������� 2mol Br2
D. X ����ʽΪ C24H17O8Cl���Ƿ����廯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� ���Ȼ���(ICl3)��ҩ��ϳ�����;�dz��㷺�����۵㣺33�����е㣺73����ʵ���ҿ�����ͼ��ʾװ����ȡICl3 ��

��1������a �������� ��
��2���Ʊ�����ѡ�õ�ҩƷΪƯ�۹���[��Ҫ�ɷ�ΪCa(ClO)2]��Ũ���ᣬ�йط�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��B(����ƿ)�������ڳ��ӣ�Ҳ�ǰ�ȫƿ���ܼ��ʵ�����ʱװ��C���Ƿ����˶�������������������ʱB������ ��
��4���Լ�XΪ ��
��5�������뵥�ʵ������¶��Ե���70���·�Ӧ����װ��D���˵ļ��ȷ�ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������й�����������Һ��������ȷ����(������Һ���ʱ����仯)
�� | �� | �� | �� | |
pH | 12 | 12 | 2 | 2 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. ���٢��зֱ�����Ȼ�茶���������Һ��pHֵ������
B. �ֱ��������������ˮϡ��100����������Һ��pH����>��
C. ���٢�����Һ�������Ϻ�������Һ������
D. ����Һ������Һ��������������Ϻ�������ҺpH��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л������У���ɵĻ�ѧԪ���������ٵ�һ����
A. ��ά�غ�֬�� B. ά���غ��ȵ���
C. ���Ǻͺ��� D. ��ԭ����֬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������X��Y��Z���ֽ�������X��Y����ϡ�����У��õ������ӣ�����������X��Y����X����Z����������Һ�У�X������Z�����������ֽ����Ļ��˳����
A. Y>X>Z B. X>Y>Z C. Z>Y>X D. Z>X>Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҽ�����̷���FeSO47H2O��������ȱ����ƶѪ����Чҩ��ij��ѧ��ȤС����̷����������µ�̽����

���Ʊ���Ʒ����С���ɷ���м�����������ۡ�����ͭ�������������ʣ�������ͼ��ʾװ���Ʊ�FeSO47H2O���壬�������£�
��1��Ԥ�������Ƚ�����м���뵽����Na2CO3��Һ��ϴ�ӣ�Ŀ����_________________��Ȼ����м��ˮϴ��2��3�顣
��2����ϴ�Ӻ�ķ���м���뵽Բ����ƿ�С���ϡ������з�ӦǰҪ����ͨ��N2��ͨ��N2��������____________________��
��3����������ϡ���ᣬ�����¶�50�桫80��֮�䣬��ַ�Ӧ��Բ����ƿ��ʣ��Ĺ���Ϊ_________��
��4����ȡ��Ʒ�������裨3���з�Ӧ��Ļ������������������ˮ�����ȹ��ˡ�����Һ____________���˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ��ܱձ����Ʒ��
���ⶨFeSO47H2O������
��1����ȡ������Ʒ10.0g������������ϡ�����У����100mL��Һ����Ҫ����������ƽ�����������ձ�����Ͳ�⣬����Ҫ�������У����������ƣ�___________��_____________��
��2������Һ��ȷ��ȡ25.00mL��Һ������ƿ�У���0.1000mol/LKMnO4����Һ�ζ�����ζ��յ���жϷ�����__________________________________��
��3����ͬ���ķ����ζ�3�Σ�ƽ������10.00mL��Һ������Ʒ��FeSO47H2O����������Ϊ_________������֪Mr��FeSO47H2O��=278����
��4�����������ƫС�����������ñ���Һ�ζ�ʱ������___������ţ��������¡�
A����ƿ����ˮϴ��δ���Ҳδ�ô���Һ��ϴ
B����ʽ�ζ���δ�ñ�Һ��ϴ��ֱ������ʢװ����Һ
C���ζ��յ�ʱ�����Ӷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��������ǻ��������������������Ӧ�ù㷺��

��1��P4S3����������������ӽṹ��ͼ1��ʾ��
��P4S3��������ԭ�ӵ��ӻ��������Ϊ ________________��
��ÿ��P4S3�����к��µ��ӶԵ���ĿΪ_________________��
��2�������۵�Ϊ2000�棬���뾧��軥Ϊ�ȵ����壬���������ṹ��ͼ2��ʾ��
�����������������������������Ϊ_______________��
��ͼ��A���B���ԭ�����������ͼ��ʾ�� ��C���ԭ���������Ϊ_____________��
��3��Fe3+��Co3-��N3-��CN-�ȿ��γ�������ӡ�
��C��N��O�ĵ�һ����������Ϊ____________����ԭ����________________________________��
��K3[Fe(CN)6]�����ڼ���Fe2+�� lmol[Fe(CN)6]3-�����к��ЦҼ�����ĿΪ__________________��
��[Co(N3)(NH3)5]SO4��Co����λ��Ϊ_____________��
��4��������FeF3�۵����1000�棬��Fe(CO)5���۵�ȴ����0��, FeF3�۵�Զ����Fe(CO)5�Ŀ���ԭ����__________________________________________________��
��5��ij�ִ��Ե���������Ľṹ��ͼ 3��ʾ���û�����Ļ�ѧʽΪ_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com