
 Åš¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓ¦ÓĆ¹ć·ŗ£®
Åš¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śÖŠÓ¦ÓĆ¹ć·ŗ£®·ÖĪö £Ø1£©ÅšŌ×ÓµÄŌ×ÓŠņŹżĪŖ5£¬¹²ÅŲ¼2øöÄÜ²ć£¬øł¾ŻÄÜĮæ×īµĶŌĄķŹéŠ“×īĶā²ćµē×ÓÅŲ¼Ź½£»²»Ķ¬Äܼ¶ÉĻµÄµē×Ó¾ßÓŠ²»Ķ¬µÄÄÜĮ棻
£Ø2£©øł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪČ·¶ØH3O+æռ乹ŠĶ”¢ŅõĄė×ÓÖŠÖŠŠÄŌ×ÓŌӻƷ½Ź½£®
£Ø3£©øł¾ŻµČµē×ÓĢåøÅÄīæÉÖŖ£¬Ō×ÓŹżŗĶ¼Ūµē×ÓŹż¶¼ĻąµČµÄĪ¢Į£»„ĪŖµČµē×ÓĢ壬¾Ż“Ė·ÖĪö£»
£Ø4£©ÓÉŃōĄė×ÓŗĶŅõĄė×Ó¹¹³ÉµÄ»ÆŗĻĪļĪŖĄė×Ó»ÆŗĻĪļ£»
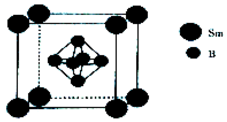
£Ø5£©øĆ¾§°ūÖŠSmŌ×ÓøöŹżĪŖ8”Į$\frac{1}{8}$=1”¢BŌ×ÓøöŹż6£¬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©ÅšŌ×ÓŗĖĶāµē×ÓŹżĪŖ5£¬»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼ĪŖ1S12S22P1£¬²»Ķ¬Äܼ¶ÉĻµÄµē×Ó¾ßÓŠ²»Ķ¬µÄÄÜĮ棬¹ŹµŖŌ×ÓŗĖĶāÓŠ3ÖÖ²»Ķ¬ÄÜĮæµÄµē×Ó£¬
¹Ź“š°øĪŖ£ŗ1s22s22p1£»3£»
£Ø2£©H3O+ÖŠOŌ×Ó¼Ū²ćµē×Ó¶ŌøöŹż=3+$\frac{1}{2}$£Ø6-1-3”Į1£©=4ĒŅŗ¬ÓŠŅ»øö¹Āµē×Ó¶Ō£¬ĖłŅŌĪŖČż½Ē׶ŠĪ½į¹¹£¬ŅõĄė×ÓÖŠÖŠŠÄŌ×ÓBŌ×Óŗ¬ÓŠ4øö¦Ņ¼üĒŅ²»ŗ¬¹Āµē×Ó¶Ō£¬ĖłŅŌBŌ×Ó²ÉÓĆsp3ŌӻƷ½Ź½£¬
¹Ź“š°øĪŖ£ŗČż½Ē׶ŠĪ£»sp3£»
£Ø3£©øł¾ŻµČµē×ÓĢåøÅÄīæÉÖŖ£¬Ō×ÓŹżŗĶ¼Ūµē×ÓŹż¶¼ĻąµČµÄĪ¢Į£»„ĪŖµČµē×ÓĢ壬BH4-Ąė×ÓÖŠÓŠ5øöŌ×Ó£¬¼Ūµē×ÓŹżĪŖ8£¬ĖłŅŌÓėBH4-Ąė×Ó»„ĪŖµČµē×ÓĢåµÄŅ»ÖÖ·Ö×ÓĪŖCH4”¢SiH4µČ£¬
¹Ź“š°øĪŖ£ŗCH4£Ø»ņSiH4£©£»
£Ø4£©EminBF4ÓÉÓŠ»śĪļŃōĄė×Ó[Emin]+ŗĶ[BF4]-¹¹³É£¬ÓÉĄė×Ó¹¹³ÉµÄ»ÆŗĻĪļŹōÓŚĄė×Ó»ÆŗĻĪļ£¬
¹Ź“š°øĪŖ£ŗĄė×Ó£»
£Ø5£©øĆ¾§°ūÖŠSmŌ×ÓøöŹżĪŖ8”Į$\frac{1}{8}$=1”¢BŌ×ÓøöŹż6£¬M=£Ø150+6”Į11£©g/mol=216g/mol£¬¾§°ū³£Źża=n pm£¬øĆ¾§ĢåĆܶȦŃ=$\frac{m}{V}$=$\frac{\frac{216}{{N}_{A}}}{£Øn”Į1{0}^{-10}£©^{3}}$=$\frac{2.16”Į1{0}^{32}}{{n}^{3}”Į{N}_{A}}$g•cm-3£¬
¹Ź“š°øĪŖ£ŗ$\frac{2.16”Į1{0}^{32}}{{n}^{3}”Į{N}_{A}}$£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹ½į¹¹ŗĶŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬Éę¼°Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½”¢Ąė×ÓµÄæռ乹ŠĶ”¢¾§°ū¼ĘĖćµČÖŖŹ¶µć£¬²ąÖŲæ¼²éѧɜ·ÖĪöÅŠ¶Ļ”¢¼ĘĖć¼°æÕ¼äĻėĻóÄÜĮ¦£¬ĄūÓĆ¾łĢÆ·Ö”¢¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪ”¢¹¹ŌģŌĄķµČÖŖŹ¶µć·ÖĪö½ā“š£¬¾§°ū¼ĘĖćŹĒøĆĢāµÄÄŃµć£¬ĢāÄæÄѶČÖŠµČ£®


 דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø
דŌŖ·»Č«³ĢĶ»Ęʵ¼Į·²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
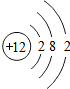 £¬Ęäµē×ÓĖłÕ¼¾ŻµÄµē×Ó²ćÖŠ£¬ÄÜĮæ×īøߵďĒM²ć£ØĢī·ūŗÅ£©£®
£¬Ęäµē×ÓĖłÕ¼¾ŻµÄµē×Ó²ćÖŠ£¬ÄÜĮæ×īøߵďĒM²ć£ØĢī·ūŗÅ£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 6£ŗ5 | B£® | 3£ŗ2 | C£® | 15£ŗ2 | D£® | 3£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģæ¾ßÓŠĒ滹ŌŠŌ£¬øßĪĀĻĀÄܽ«¶žŃõ»Æ¹č»¹ŌĪŖ¹č | |
| B£® | Ģ¼ĖįĒāÄĘÄÜÓė¼ī·“Ó¦£¬æÉÓĆ×÷Ź³Ę·µÄÅīĖɼĮ | |
| C£® | ÅØĮņĖį¾ßÓŠĪüĖ®ŠŌ£¬æÉÓĆÓŚøÉŌļ°±Ęų”¢¶žŃõ»ÆĢ¼µČĘųĢå | |
| D£® | ĀĮÄÜÖĆ»»³öŃõ»ÆĢśÖŠµÄĢś£¬øÖĢś¹¤ŅµĄūÓĆĀĮČČ·“Ó¦Ņ±Į¶Ģś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Šæ¼°Ęä»ÆŗĻĪļӊ׏ć·ŗµÄÓ¦ÓĆ£®Ēė»Ų“šÓŠ¹ŲŠæ¼°Ęä»ÆŗĻĪļµÄÓŠ¹ŲĪŹĢā£®
Šæ¼°Ęä»ÆŗĻĪļӊ׏ć·ŗµÄÓ¦ÓĆ£®Ēė»Ų“šÓŠ¹ŲŠæ¼°Ęä»ÆŗĻĪļµÄÓŠ¹ŲĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØŃĪĖį¾ßÓŠ»Ó·¢ŠŌ | B£® | ÅØĮņĖį¾ßÓŠĶŃĖ®ŠŌ | ||
| C£® | ÅØĮņĖįŗĶÅØŃĪĖįÖŠµÄĖ®×÷Óƶų·ÅČČ | D£® | ÅØĮņĖįÓŠĪüĖ®ŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2Ļī | B£® | 3Ļī | C£® | 4Ļī | D£® | 5Ļī |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com