
(9·Ö)ŅŃÖŖA”¢B”¢C”¢DŗĶE¶¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠĒ°36ŗŵÄŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£A ÓėĘäĖū4ÖÖŌŖĖŲ¼Č²»ŌŚĶ¬Ņ»ÖÜĘŚÓÖ²»ŌŚĶ¬Ņ»Ö÷×唣BŗĶCŹōĶ¬Ņ»Ö÷×壬DŗĶEŹōĶ¬Ņ»ÖÜĘŚ£¬ÓÖÖŖEŹĒÖÜĘŚ±ķÖŠ1”Ŗ18ĮŠÖŠµÄµŚ7ĮŠŌŖĖŲ”£DµÄŌ×ÓŠņŹż±ČEŠ”5£¬DøśBæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļĘ侧°ū½į¹¹ČēÓŅĶ¼”£ 
 £Ø1£©AµÄŌŖĖŲ·ūŗÅŹĒ £»EŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ £¬ĖüµÄ+2¼ŪĄė×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø1£©AµÄŌŖĖŲ·ūŗÅŹĒ £»EŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ £¬ĖüµÄ+2¼ŪĄė×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£  £Ø2£©BµÄĒā»ÆĪļµÄ¾§ĢåĄąŠĶŹĒ ¾§Ģ壬BµÄĒā»ÆĪļÓėCµÄĒā»ÆĪļĻą±Č£¬·Ö×Ó¼«ŠŌ½Ļ“óµÄŹĒ £ØŠ“»ÆѧŹ½£©”£
£Ø2£©BµÄĒā»ÆĪļµÄ¾§ĢåĄąŠĶŹĒ ¾§Ģ壬BµÄĒā»ÆĪļÓėCµÄĒā»ÆĪļĻą±Č£¬·Ö×Ó¼«ŠŌ½Ļ“óµÄŹĒ £ØŠ“»ÆѧŹ½£©”£ £Ø3£©“ÓĶ¼ÖŠæÉŅŌ擳ö£¬DøśBŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ £»øĆĄė×Ó»ÆŗĻĪļ¾§ĢåµÄĆܶČĪŖag”¤cm-3£¬Ōņ¾§°ūµÄĢå»żŹĒ £ØÖ»ŅŖĒóĮŠ³öĖćŹ½£©”£
£Ø3£©“ÓĶ¼ÖŠæÉŅŌ擳ö£¬DøśBŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ £»øĆĄė×Ó»ÆŗĻĪļ¾§ĢåµÄĆܶČĪŖag”¤cm-3£¬Ōņ¾§°ūµÄĢå»żŹĒ £ØÖ»ŅŖĒóĮŠ³öĖćŹ½£©”£
(1)H µŚĖÄÖÜĘŚ”¢µŚ¢÷B×壻1s22s22p63s23p63d5£»£Ø2£©·Ö×Ó HF£Ø3£©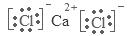
4”Į78g/mol/(6.02”Į1023”Įag”¤cm-3)
½āĪöŹŌĢā·ÖĪö£ŗøł¾ŻĢāŅāæÉĶĘÖŖø÷ÖÖŌŖĖŲ·Ö±šŹĒ£ŗAŹĒH£»BŹĒF£»CµÄCl£»DµÄCa£»FŹĒMn”£¢ÅAµÄŌŖĖŲ·ūŗÅŹĒH£»EŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒµŚĖÄÖÜĘŚ”¢µŚ¢÷B×壻ĖüµÄ+2¼ŪĄė×ӵĵē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d5£»¢ĘBµÄĒā»ÆĪļµÄ¾§ĢåĄąŠĶŹĒ·Ö×Ó¾§Ģ壻F”¢ClŹĒĶ¬Ņ»Ö÷×åµÄŌŖĖŲ£¬ÓÉÓŚŌŖĖŲµÄ·Ē½šŹōŠŌF>Cl£¬ŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬ĘäĒā»ÆĪļµÄĪČ¶ØŠŌ¾ĶŌ½Ē棬ĖłŅŌBµÄĒā»ÆĪļÓėCµÄĒā»ÆĪļĻą±Č£¬·Ö×Ó¼«ŠŌ½Ļ“óµÄŹĒHF£»¢Ē “ÓĶ¼ÖŠæÉŅŌ擳ö£¬ŌŚDøśBŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļÖŠŗ¬ÓŠF£ŗ8£»ŗ¬ÓŠCa:8”Į1/8+6”Į1/2=4,ĖłŅŌ»ÆѧŹ½µÄCaF2£¬øĆ»ÆŗĻĪļŹĒĄė×Ó»ÆŗĻĪļ£¬Ęäµē×ÓŹ½ĪŖ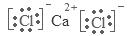 ;ŌŚĆæøö¾§°ūÖŠŗ¬ÓŠ4øöCaF2£¬ÓÉÓŚ¾§ĢåµÄĆܶČ
;ŌŚĆæøö¾§°ūÖŠŗ¬ÓŠ4øöCaF2£¬ÓÉÓŚ¾§ĢåµÄĆܶČ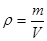 £»ĖłŅŌ
£»ĖłŅŌ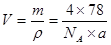 =4”Į78g/mol/(6.02”Į1023”Įag”¤cm-3)”£
=4”Į78g/mol/(6.02”Į1023”Įag”¤cm-3)”£
æ¼µć£ŗæ¼²éŌŖĖŲµÄĶʶĻ”¢ŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ”¢Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½”¢µē×ÓŹ½µÄŹéŠ“”¢ĪļÖŹµÄĪČ¶ØŠŌµÄ±Č½Ļ”¢¾§°ūĢå»żµÄ¼ĘĖćµÄÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ
| A£®¾§ĢåÓė·Ē¾§ĢåµÄøł±¾Ēų±šŌŚÓŚŹĒ·ń¾ßÓŠ¹ęŌņµÄ¼øŗĪĶāŠĪ |
| B£®¾§Ģå¾ßÓŠĪļĄķŠŌÖŹø÷ĻņŅģŠŌ |
| C£®¾§Ģ唢·Ē¾§Ģå¾ł¾ßÓŠ¹Ģ¶ØµÄČŪµć |
| D£®Óɲ£Į§ÖĘ³É¹ęŌņµÄ²£Į§ĒņĢåĻÖĮĖ¾§ĢåµÄ×Ō·¶ŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŠĪ³ÉĪļÖŹŹĄ½ē¶ąŃłŠŌµÄŌŅņÓŠ£ŗ( )
¢ŁŌŖĖŲÖÖĄą ¢ŚĶ¬Ī»ĖŲ ¢Ū»Æѧ¼ü³É¼ü·½Ź½ ¢ÜĶ¬·ÖŅģ¹¹ĻÖĻó ¢ŻĶ¬ĖŲŅģŠĪĻÖĻó
| A£®½ö¢Ł¢Ś¢Ū | B£®½ö¢Ś¢Ü¢Ż |
| C£®½ö¢Ł¢Ū | D£®¢Ł¢Ś¢Ū¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©Ń”ŌńŅŌĻĀĪļÖŹĢīŠ“ĻĀĮŠæÕ°×£ŗ
| A£®ĒāŃõ»ÆÄĘ | B£®Ńõ»ÆĆ¾ | C£®He | D£®¶žŃõ»Æ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©Ė®ŌŚ²»Ķ¬µÄĪĀ¶ČŗĶŃ¹ĒæĢõ¼žĻĀæÉŅŌŠĪ³É11ÖÖ²»Ķ¬½į¹¹µÄ¾§Ģ壬ĆܶȓӱČĖ®ĒįµÄ0.92 g”¤cm£3µ½Ō¼ĪŖĖ®µÄŅ»±¶°ė”£±łŹĒČĖĆĒĘł½ńŅŃÖŖµÄÓÉŅ»ÖÖ¼ņµ„·Ö×Ӷѻż³ö½į¹¹»ØŃł×ī¶ąµÄ»ÆŗĻĪļ”£ĘäÖŠ±ł£¢÷µÄ¾§Ģå½į¹¹ĪŖŅ»øöČēĻĀĶ¼ĖłŹ¾µÄĮ¢·½¾§°ū£¬ĆæøöĖ®·Ö×ÓæÉÓėÖÜĪ§____________øöĖ®·Ö×ÓŅŌĒā¼ü½įŗĻ£¬¾§ĢåÖŠ£¬1 molĖ®æÉŠĪ³É________ molĒā¼ü”£
£Ø2£©ŅŃÖŖĻĀĮŠŌŖĖŲµÄµēøŗŠŌŹż¾Ż£ŗHĪŖ2.1£¬OĪŖ3.5£¬FĪŖ4.0”£OF2ÓėĖ®µÄĮ¢Ģå½į¹¹ĻąĖĘ£¬µ«Ė®·Ö×ӵļ«ŠŌ±ČOF2ĒæµĆ¶ą£¬ĘäŌŅņÓŠ£ŗ¢ŁOF2ÖŠŃõŌ×ÓÉĻÓŠĮ½¶Ō¹Ā¶Ōµē×Ó£¬µÖĻūĮĖF”ŖO¼üÖŠ¹²ÓƵē×Ó¶ŌĘ«ĻņF¶ų²śÉśµÄ¼«ŠŌ£»¢Ś“ÓµēøŗŠŌÉĻæ“£¬___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖÄņĖŲµÄ½į¹¹Ź½ĪŖ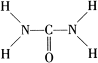 £ŗ ÄņĖŲæÉÓĆÓŚÖĘÓŠ»śĢś·Ź£¬Ö÷ŅŖ“ś±ķĪļÓŠČżĻõĖįĮłÄņĖŲŗĻĢś£Ø¢ó£©£¬»ÆѧŹ½ĪŖ£ŪFe£ØH2NCONH2£©6£Ż£ØNO3£©3”£
£ŗ ÄņĖŲæÉÓĆÓŚÖĘÓŠ»śĢś·Ź£¬Ö÷ŅŖ“ś±ķĪļÓŠČżĻõĖįĮłÄņĖŲŗĻĢś£Ø¢ó£©£¬»ÆѧŹ½ĪŖ£ŪFe£ØH2NCONH2£©6£Ż£ØNO3£©3”£
£Ø1£©»łĢ¬Fe3+µÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ £»C”¢N”¢OČżÖÖŌŖĖŲµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ ”£
£Ø2£©ÄņĖŲ·Ö×ÓÖŠNŌ×ÓµÄŌӻƷ½Ź½ŹĒ ”£
£Ø3£©NH+4ÖŠH”ŖN”ŖH¼ü½Ē±ČNH3ÖŠH”ŖN”ŖH¼ü½Ē“ó£¬ŌŅņĪŖ ”£
£Ø4£©CO2ŗĶNH3ŹĒ¹¤ŅµÉĻÖʱøÄņĖŲµÄÖŲŅŖŌĮĻ£¬¹ĢĢ¬CO2£Øøɱł£©µÄ¾§°ū½į¹¹ČēÓŅĶ¼ĖłŹ¾”£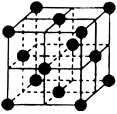
¢Ł1øöCO2·Ö×ÓÖÜĪ§µČ¾ąĄėĒŅ¾ąĄė×ī½üµÄCO2·Ö×ÓÓŠ øö”£
¢ŚNaCl¾§°ūŅ²ĪŖĆęŠÄĮ¢·½½į¹¹£¬ŅŃÖŖNaCl¾§ĢåĆܶČĪŖ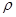 g”¤cm-3£¬NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌņNaCl¾§°ūĢå»żĪŖ cm3
g”¤cm-3£¬NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌņNaCl¾§°ūĢå»żĪŖ cm3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĖµ·Ø»ņ±ķŹ¾·½·ØÕżČ·µÄŹĒ£Ø £©
| A£®µČĪļÖŹµÄĮæµÄĮņÕōĘųŗĶĮņ·Ū·Ö±šĶźČ«Č¼ÉÕ£¬ŗóÕ߷ųöČČĮæ¶ą |
| B£®ÓÉC(ŹÆÄ«)”śC(½šøÕŹÆ) ¦¤H=£«11.9kJ”¤mol-1æÉÖŖ£¬½šøÕŹÆ±ČŹÆÄ«ĪČ¶Ø |
| C£®ŌŚ25”ę£¬101kPaŹ±£¬2gĒāĘųĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö285.8kJČČĮ棬ŌņĒāĘųČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½æɱķŹ¾ĪŖ£ŗ2H2(g)£«O2(g)=2H2O(l) ¦¤H=£571.6kJ”¤mol-1 |
| D£®H£«(aq)£«OH£(aq)= H2O(l) ¦¤H=£57.3kJ”¤mol-1£¬Čō½«ŗ¬0.5molH2SO4µÄÅØĮņĖįÓėŗ¬1molNaOHµÄĒāŃõ»ÆÄĘČÜŅŗ»ģŗĻ£¬·Å³öµÄČČĮæµČÓŚ57.3kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
»Æѧ·“Ó¦A2 £« B2 £½ 2ABµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ( )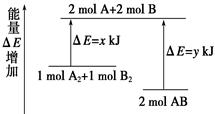
| A£®øĆ·“Ó¦ŹĒĪüČČ·“Ó¦ |
| B£®¶ĻĮŃ1 mol A”ŖA¼üŗĶ1 mol B”ŖB¼üÄܷųöx kJµÄÄÜĮæ |
| C£®¶ĻĮŃ2 mol A”ŖB¼üŠčŅŖĪüŹÕy kJµÄÄÜĮæ |
| D£®2 mol ABµÄ×ÜÄÜĮæøßÓŚ1 mol A2ŗĶ1 mol B2µÄ×ÜÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĪļÖŹ½į¹¹ĄķĀŪÖø³ö£ŗ½šŹō¾§ĢåÖŠ½šŹōŃōĄė×ÓÓė×ŌÓɵē×ÓÖ®¼äµÄĒæĮŅµÄĻą»„×÷ÓĆ£¬½Š½šŹō¼ü”£½šŹō¼üŌ½Ē棬Ę佚ŹōµÄÓ²¶ČŌ½“ó£¬ČŪ”¢·ŠµćŌ½øߣ¬ĒŅ¾ŻŃŠ¾æ±ķĆ÷£¬Ņ»°ćĖµĄ“½šŹōŌ×Ó°ė¾¶Ō½Š”£¬¼Ūµē×ÓŹżŌ½¶ą£¬Ōņ½šŹō¼üŌ½Ē攣ÓÉ“ĖÅŠ¶ĻĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø £©”£
| A£®Ć¾µÄÓ²¶ČŠ”ÓŚĀĮ |
| B£®Ć¾µÄČŪ”¢·ŠµćµĶÓŚøĘ |
| C£®Ć¾µÄÓ²¶Č“óÓŚ¼Ų |
| D£®øʵÄČŪ”¢·ŠµćøßÓŚ¼Ų |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com