
ij��ѧ��ȤС������ͼװ�õ��CuSO4��Һ���ⶨͭ����Է���������
��1����ʵ���вⶨ�ڱ�״���·ų������������VL��A����ֱ����Դ��__________ (�����������������
��2����ʼһ��ʱ�����U�ι��пɹ۲쵽��������____________________________��
�������ӷ���ʽΪ ��
��3��ʵ���л���ⶨ��������_______________����д��ţ���
��A������������m g ��B������������m g
��4������ʵ������б�Ҫ����____________������ĸ����
| A���������ǰ�缫������ |
| B�����缫�ں�ɳ���ǰ������������ˮ��ϴ |
| C�����µ������е缫��������ͭ������ϴ������ |
| D���缫�ں�ɳ��صIJ����б��밴����ɡ�����һ�ٺ��һ�ٳ��ء����� |
��1���� ��2��A����֣�B�������ݲ�������Һ��ɫ��dz2Cu2����2H2O 2Cu��O2����4H��
2Cu��O2����4H��
��3���� ��4��ABDE ��5��11.2m/V ��6��CH3OH��8OH����6e��=CO32����6H2O
���������������1�����O2�����ΪVL��A��Ϊ������
��2������ΪA����֣�B�������ݲ�������Һ��ɫ��dz���缫��ӦʽΪ2Cu2����2H2O 2Cu��O2����4H����4���缫��������Cu����������Ҫ���£�ֻ��Ҫ�Ƴ�A�����������������ʲ���ҪC��IJ�����
2Cu��O2����4H����4���缫��������Cu����������Ҫ���£�ֻ��Ҫ�Ƴ�A�����������������ʲ���ҪC��IJ�����
��5�� ��ͭ��Ħ������ΪMmol/g 2Cu��O2
2M 1
m V/22.4
���M=11.2m/V
���㣺������ԭ������������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾװ�����ӣ�X��Y��Ϊ���Ե缫����ش��������⣺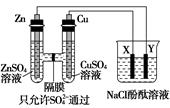
��1��ZnΪ________����
��2�����Ӻ�װ�ú��ձ��е���Һ������Ӧ�����ӷ���ʽ��___________��
��3��ͼ��ͨ����Ĥ��SO42-��________������ҡ�������Ǩ�ƣ�Y�����丽�����ֵ�������________��
��4�������£���Zn����������32.5 gʱ��X����������8.4 L����״����������ʱ�ձ�����Һ�����Ϊ500 mL�����ʱ�ձ�����Һ��pH��________�����������ɵ���������ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��⼼�����д���Ч��������Ͷ���١����з��õ͵��ŵ㡣��̼����������������̼����֮���γ���������ϸԭ��أ���Щϸ�����������Һ�з����绯ѧ��Ӧ����Һ���ܽ�һ�������������������ĸ�ʴЧ�ʣ���˿���ͨ��������ǿ������Ч����ʵ��װ����ͼ��
��1��ʵ��ǰ������м��5%NaOH��ϴ10���ӣ�Ŀ���Ǣ� ���� ��
��2�����������£���������Һ�У���װ���з����ĵ缫��ӦΪ������ ��̼�� ��
��3��ͨ����̼��ⷨ���ԶԺ�����Ʒ�ˮ���д�����ij��ѧ�о�С���о�����̼�ȡ���Ӧͣ��ʱ��Դ���Ч����Ӱ�죬������£�

ͨ��������ȷ����ѹ��յ���̼��Ϊ ����Ӧͣ��ʱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����FeCl3��Һ��ʴӡˢ��·ͭ��Ļ�ѧ����ʽΪ�� �������÷�Ӧ��Ƴ���ͼ��ԭ��أ�����ͼ����ɱ�ע��
��2��ʵ��֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ���������з�Ӧ��
��C(s)+H2O(g)=CO(g)+H2(g) ��H>0��
��2H2(g)+O2(g)=2H2O(1) ��H<0 ��
��NaOH(aq)+HC1(aq)=NaC1(aq)+H2O(1) ��H<0 ��
������Ӧ������Ƴ�ԭ��ص��� ������ţ���������KOH��ҺΪ�������Һ��������ѡ��Ӧ��Ƶ�ԭ����为����ӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1������ˮ�����׳ư�˾ƥ�֣���һ����ʷ�ƾõĽ�����ʹҩ������ˮ����Ľṹ��ʽΪ ��
��
��������ˮ����Ĵ�Ʒ��ijͬѧ���кͷ��ⶨ��Ʒ���ȣ�ȡa g��Ʒ�ܽ���V1 mL1mol/L��NaOH��Һ�У�����ʹ����ˮ����ˮ�⣬����1 mol/L������ζ�������NaOH�����ζ��յ�ʱ��������V2 mL��
��д������ˮ������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
�ڼ������Ʒ����Ϊ ��ֻ���г��������ʽ�����ػ�������ˮ������Է�������Ϊ180����
��2���״�ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߡ������ܶȸߵ��ŵ㡣����֪������ֱ��ȼ�ϵ�������ܶ�E =8��39 kW��h��kg-1)��
���������Ϊ���ԣ��״�ֱ��ȼ�ϵ�صĸ�����ӦΪ ��
�ڸõ�ص����������ѹΪ1��20 V�������ܶ�E = (��ʽ���㡣�����ܶ� = ���������ܣ�ȼ��������lkW��h = 3��6��106J,һ�����ӵĵ���=1��6��10��19C)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���հ�ɽ��������������ɽ���ڣ�������½���ɽһ�������������׳ơ������͡�������������Ҫ�Ŀ���֮һ�������б���Ϊ����ʯ�����������ߣ�����ұ���̸ֵ���Ҫԭ�ϡ�����ʯ��Ҫ�ɷ��д�����Fe3O4��������FeCO3���̿�MnO2��MnCO3��ʯ��Mg3Si3O7(OH)4�ȡ���ҵ�Ͻ�����ʯ����������������Ĥ��ⷨ���¼�����ȡ�����̲��Ƶ���ɫ��Чˮ��������K2FeO4������ҵ�������£�
��1����ҵ��Ϊ���ϡ�����ȡЧ��һ���ȡ�Ĵ�ʩ�ǣ�����д���ַ�����
�� ��
��2��ʯ��ѧʽΪMg3Si3O7(OH)4Ҳ���Ա�ʾ����������ʽ�����������ʽΪ ��
��3����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Al3+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 2.7 | 3.7 | 7.0 | 7.8 | 9.3 |
| ��ȫ������pH | 3.7 | 4.7 | 9.6 | 9.8 | 10.8 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Ӧ�з�Ӧ�����������У�FeCl2��FeCl3��CuCl2��Cu��
��1����������Ӧ��Ƴɵ�ԭ�����ͼ����ʾ����ش��������⣺

��ͼ��X��Һ��_____________________________��
��ʯī�缫�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
��ԭ��ع���ʱ�������е�____________(�K������Cl����)���Ͻ���X��Һ�С�
��2����������Ӧ��Ƴɵĵ�����ͼ����ʾ�����ձ��н��������ӵ����ʵ��������ת�Ƶ����ʵ����ı仯��ϵ��ͼ������ش��������⣺
��M��__________����
��ͼ���еĢ�����______________�ı仯��
�۵�����ת��Ϊ2 molʱ�������ձ��м���________ L 5 mol��L��1 NaOH��Һ����ʹ���еĽ��������ӳ�����ȫ��
��3��������Ҫ�������������(Na2FeO4)��һ����������ˮ�����������кܶ��ŵ㡣
�ٸ���������������֮һ�ǵ�ⷨ����ԭ��ΪFe��2NaOH��2H2O=Na2FeO4��3H2��������ʱ�����ĵ缫��Ӧʽ��__________________________________��
�ڸ���������������֮������ǿ���Խ�������NaClO����Fe(OH)3���ɸ������ơ��Ȼ��ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪ_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ������ȤС���ö��Ե缫��ⱥ��ʳ��ˮ��������Mg2+����ϵ��̽����װ����ͼ��ʾ��
��1�����ʱ����ͬѧ���ֵ缫a������Һ���ֻ��ǣ��������ӷ���ʽ��ʾԭ��
___________________________________________________________________________________��
��2��һ��ʱ�������ΪC����Һ���ܳ��ֵ�������_________________________________��������
�ӷ���ʽ��ʾԭ��______________________________________________________��
��3�����ŷ�Ӧ�Ľ��У���ȤС���ͬѧ�Ƕ��ر�ע�D����Һ��ɫ����ȥ�����Ƕ���Һ��ɫ��ȥ��Ҫԭ����������¼��裬������ɼ������
����һ��B���ݳ�������ˮ��Ӧ���ɵ�������ǿ�����ԣ�ʹ��ɫ����ȥ��
�������______________________________________________________________________��
��4���������ʵ����֤��������һ��д��ʵ�鲽�輰���ۣ�_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס������ص缫���϶���������̼��������ͼ������ش��������⣺ 
(1)�������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е� �����ҳ��е� ����
�����ҳ��������ĵ缫��Ӧʽ�� ��
(2)�������о�ʢ�ű���NaCl��Һ��
��д���ҳ��з����ܷ�Ӧ�����ӷ���ʽ ��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5:1�������������ᡣ�÷�Ӧ�Ļ�ѧ����ʽΪ ��
�����ҳ�ת��0.02mol���Ӻ�ֹͣʵ�飬������Һ�������200mL������Һ���Ⱥ��pH= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com