
ij������A������ʽΪC8H10��ij����������X������ʽΪC15H14O3����ʹFeCl3��Һ ����ɫ��J��������������Ϊ��λ��ȡ��������һ�������������µ�ת����ϵ��������ȥ����
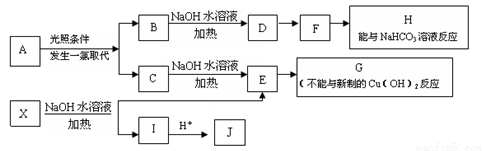
��1�����ڷ��������A��ͬ���칹�壨������A���У���ͬһƽ���ԭ�������__________����
��2��J�������ĺ��������ŵ�����Ϊ__________��
��3��E��H��Ӧ�Ļ�ѧ����ʽ��____________________________________��
��4��B��C�Ļ������NaOH�Ҵ���Һ�м��ȿ�������ͬһ���л���M����MΪ����ϳɵĸ߷��ӻ������������_________________________��
��5����֪J�ж���ͬ���칹�壬д��һ�ַ����������ʵ�J��ͬ���칹��Ľṹ��ʽ������FeCl3��Һ��������ɫ����������Cu(OH)2����Һ���ò�����ɫ�������۱����ϵ�һ±������2�֡�
______________________________________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ������и߶���ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��VSEPRģ��Ԥ�����з��ӻ����ӵ�����ṹ��������ȷ���� �� �� ��
A. H2O��BeCl2Ϊ���Σ�V�Σ� B. CS2��SO2Ϊֱ����
C. SO3��CO Ϊƽ�������� D. BF3��PCl3Ϊ������
Ϊƽ�������� D. BF3��PCl3Ϊ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ�ϳ��и����ڶ��θ߿���Ӧ�Կ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���H2C2O4��Һ����μ���NaOH��Һ��������Һ��H2C2O4��HC2O4-��C2O42-�������ʵ����������ֲ�ϵ������pH�仯�Ĺ�ϵ����ͼ��ʾ����ͼΪ��ͬŨ��NaHC2O4��Һ�в�����Ũ�ȡ����б����������
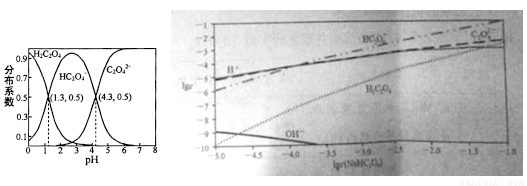
A. HC2O4- H++C2O42- K=1��10-4.3
H++C2O42- K=1��10-4.3
B. �������ʵ�����NaHC2O4��Na2C2O4����ˮ�У�������ҺpHǡ��Ϊ4.3
C. NaHC2O4��Һ��һ������c(C2O42-)>c(H2C2O4)��ͬʱc(H+)>c(OH-)
D. ��0.1mol/L NaHC2O4��Һ�и�����Ũ�ȴ�С��ϵΪ��c(Na+)>c(HC2O4-)>c(C2O42-)>c(H2C2O4)> c(H+)>c(OH-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�Ͼ��С��γ��и����ڶ���ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ʾ���Ӧ�����������
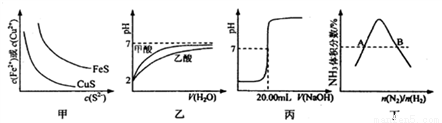
A. ͼ�ױ�ʾһ���¶���FeS��CuS�ij����ܽ�ƽ�����ߣ���Ksp(FeS)>Ksp(CuS)
B. ͼ�ұ�ʾpH=2�ļ�����������Һϡ��ʱ��pH�仯���ߣ������ԣ�����<����
C. ͼ����ʾ��0.1000 mol��L-lNaOH��Һ�ζ�25.00 mL����ĵζ����ߣ��� c(HCl)=0.0800 mol ��L-1
D. ͼ����ʾ��ӦN2(g)+3H2(g)  2NH3(g)ƽ��ʱNH3�����������ʼn(N2)/n(H2)�仯�����ߣ���ת���ʣ���A(H2)=��B(H2)
2NH3(g)ƽ��ʱNH3�����������ʼn(N2)/n(H2)�仯�����ߣ���ת���ʣ���A(H2)=��B(H2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�Ͼ��С��γ��и����ڶ���ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���з�Ӧ�����ӷ���ʽ��ȷ����
A. ͭ��ϡHNO3��Ӧ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
B. ����������Һ�м��������ˮ��Al3++3OH-= AlO2-+2H2O
C. ��Ag(NH3)2NO3��Һ�м������Ag(NH3)2++2H+=Ag++2NH4+
D. NaHSO4��Һ��Ba(OH)2��Һ��Ϻ���Һ�����ԣ�Ba2++OH-+H++SO42-=BaSO4��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ijʯ�ͻ�����ƷX��ת����ϵ����ͼ,�����ж���ȷ����(��)
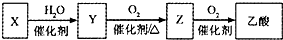
A. 0.1 mol��L-1������ҺpHΪ1 B. 1 mol Y�����������Ʒ�Ӧ��������3 mol H2
C. Z��CH3OCH3��Ϊͬ���칹�� D. Z��ͨ���ӳɷ�Ӧ����Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����л���ķ����������ǻ������ŵķ�Ӧ�����У�
��������Ӧ ��ȡ����Ӧ ����ȥ��Ӧ �ܼӳɷ�Ӧ ��ˮ�ⷴӦ��������ȷ�������
A���٢ڢ� B���ܢ� C���ڢܢ� D���ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���ѧ�ڵ�һ�ν�ѧ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
���и����еķ�Ӧ������ͬһ��Ӧ���͵���( )
A. ��������Ҵ��������������ɱ���������ˮ���Ʊ�������Ҵ�
B. �������ˮ���Ʊ������ɱ�ϩ��ˮ��Ӧ�Ʊ���
C. �ɼױ������ƶ������ױ����ɼױ������Ʊ�����
D. ���ȴ���������ȥ�ƻ���ϩ���ɱ�ϩ������1,2?�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��«���и�����һ��ģ�⿼�����ۻ�ѧ�Ծ� ���ͣ�ʵ����
̼���ơ�̼���������ճ����������г��õ������Ρ���ʵ��������ȡNaHCO3�ķ����ж��֡���
�������з����ش����⣺
����һ��ģ�ҵ�������Ƽ����ȡ����ԭ��NaCl+H2O+CO2+NH3 = NaHCO��+NH4Cl)
��һ�������Ӻ�װ�ã����������ԣ���������װ��ҩƷ��
�ڶ�������ijһװ���ȷ�����Ӧ��ֱ�����������岻�����ڢ����ܽ⣬��ͨ����һװ���в��������壬Ƭ�̺��г��ֹ��塣���������ͨ���������壬ֱ�������й��������
������������������õĻ����õ�NaHCO3���塣
���IJ�������Һ�м���������ij�����ĩ����NH4Cl����������
(1)ͼ����ʾװ�õ�����˳���ǣ�a��f��e��________��b��_________����ӿڱ��)��
(2) I��ʢ��ϡ���������������_____________��IV��Ӧѡ�õ�Һ��Ϊ________________��
(3 )�ڶ������� _____________ (����ţ�װ���ȷ�����Ӧ��
(4)���IJ������ӹ����ĩΪ________�����õľ����г��������õ�NaCl��NaHCO3(Լռ5%~8%)�������һ����ʵ�飬��ʹ�������κ��Լ���֤�����þ������NH4Cl����Ҫд������������______________________��
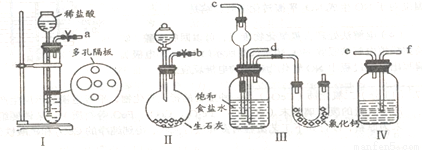
����������CO2ͨ�˱���Na2CO3��Һ��ȡNaHCO3��װ������ͼ��ʾ���������Ѽ��飬���ּг�װ���ԣ���
(5 )���ϻ�ѧƽ���ƶ�ԭ������B����Һ������_________________��
(6)��C���д�����ɫ��������ʱ��ֹͣʵ�飬��������ˡ�ϴ�ӡ����ﱸ�á�Ϊȷ������ijɷ֣�ʵ��С����Ʒ������£�ȡһ�����Ĺ��壬�����Һ��Ϊ��Һ��������屸�ã���
�ٷ���1��ȡ��Һ������Ca(OH)2��Һ��ϣ����ְ�ɫ������
ʵ��С������������ԭ�����з�������Ϊ�÷�����������������________________��
�ڷ���2��ȡ��Һ��BaCl2��Һ��ϣ����ְ�ɫ�����������������
ʵ��С����Ϊ�����д���NaHCO3,�����ӷ���ʽ��________________��
����������7��������д��һ��ʵ������ȡ����̼�����Ƶķ���������صĻ�ѧ��Ӧ����ʽ����ʾ����__________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com