
��8�֣�ÿ��2�֣�����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ���ش�
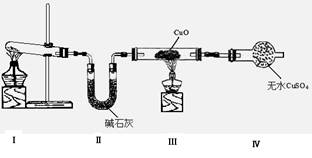
��1����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������
��װ�â�����ȡ�����Ļ�ѧ����ʽΪ ��
��װ�â��е�ʵ������ֱ��ǣ���ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
��д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
��װ�â��м�ʯ�ҵ������� ��
��2����ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+���ȶ��Ա�Cu2+��(Cu2O+2H+= Cu + Cu2+ +2H2O����
�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O��
��
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(18�֣�ÿ��2��)
�֣�
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺
��1��������ȡ�����Ļ�ѧ����ʽΪ��_____________ _________ _______��
��2����λͬѧ���������ſ������ռ���������ԭ����_________ _________��
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___ ___��(���ס������ҡ�����)��
��4�����鰱���Ƿ��ռ����ķ�����:��������������������ͽ��ۣ�
_______ _____��
��5����λͬѧ����Ϊ���ǵ�ʵ��װ�û������ڼ���̼����粒�������ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl������棿____ _(��ܡ����ܡ�)��
��
��֪���������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽΪ��
5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4
��ͼΪ��֤Ũ������ľ̿�ڼ��������£���Ӧ�������Ƿ���SO2��CO2�IJ���װ�á�
��6��ʵ��ʱ���� ������ʢ�г���ʯ��ˮ��ʵ��װ��(��a��b ���)��
��7���ɹ۲쵽Aƿ����Һ ��
��8��Cƿ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���⣺(18�֣�ÿ��2��)
�֣�
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺
��1��������ȡ�����Ļ�ѧ����ʽΪ�� ��
��2����λͬѧ���������ſ������ռ���������ԭ���� ��
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___��(���ס������ҡ�����)��
��4�����鰱���Ƿ��ռ����ķ�����:��������������������ͽ��ۣ�
��5����λͬѧ����Ϊ���ǵ�ʵ��װ�û������ڼ���̼����粒�������ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl������棿____ (��ܡ����ܡ�)��
��
��֪���������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽΪ��
5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4
��ͼΪ��֤Ũ������ľ̿�ڼ��������£���Ӧ�������Ƿ���SO2��CO2�IJ���װ�á�

��6��ʵ��ʱ���� ������ʢ�г���ʯ��ˮ��ʵ��װ��(��a��b ���)��
��7���ɹ۲쵽Aƿ��Һ ��
��8��Cƿ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ÿ��2�֣�����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ���ش�
��1����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������
��װ�â�����ȡ�����Ļ�ѧ����ʽΪ ��
��װ�â��е�ʵ������ֱ��ǣ���ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
��д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
��װ�â��м�ʯ�ҵ������� ��
��2����ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+���ȶ��Ա�Cu2+��(Cu2O+2H+= Cu + Cu2+ +2H2O ����
�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�������и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��8�֣�ÿ��2�֣�����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ���ش�
��1����С��ͬѧ�������ͼ��ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������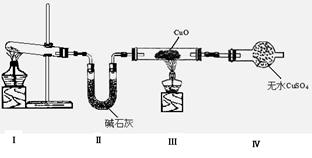
��װ�â�����ȡ���� �Ļ�ѧ����ʽΪ ��
�Ļ�ѧ����ʽΪ ��
��װ�â��е�ʵ������ֱ��ǣ���ɫCuO��Ϊ��ɫ����ɫ�� ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
��д��������CuO�� Ӧ�Ļ�ѧ����ʽ
Ӧ�Ļ�ѧ����ʽ  ��
��
��װ�â��м�ʯ�ҵ������� ��
��2����ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O����֪��Cu2O��һ�ּ����������������Һ�У�Cu+���ȶ��Ա�Cu2+��(Cu2O+2H+=" Cu" + Cu2+ +2H2O����
�������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O�� 
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com