
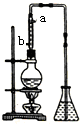
 ��Һ��
��Һ������ ������̽����ȩ���ڷ��ó��ֲַ������ԭ�Ի�������ʱ�漰��Һ����������������˲���Ҫ�㣬��ֲ����÷�Һ������ʱ����������ʱ��Ҫ����������ȴ����ȴˮ������Ϊ�ͽ��߳��ȣ����������ȩ�Ļ�ѧ�����緢��������Ӧ��̽���Ӻ���Ľṹ��
��1�����õ���ȩ��Һ������ֲ�������˷���ʱ��ѡ���Һ©��ͨ����Һ���в�����
��2������ȩ��������������CH3COOH���²�Һ�����ԣ���������ʯ����Һ�����²�Һ�Ƿ�����ԣ�
��3�����������ʱ���в��ּӺ������¶ȵ������ٻӷ��������ʹ��������ʹ֮Һ��������������ƿ�ڣ���ʹ�õ�����ȩ����������ԭ��������Ҳ�ͣ�
�����ݴ��µ�������Һ������У����Խ��ԽС��ֱ����ȫ��ʧ��˵������������Һ�����۲쵽��������������Сʱ��Ҫ�����ܴ���Һ�� ȡ�����Է�ֹ�������ݴ˴��⣮
��� �⣺��1���ȷ�������õ���C2H4O��n�����������ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ����ϲ����״Һ�壨C2H4O��n�ӷ�Һ©�����Ͽڵ�����
�ʴ�Ϊ����Һ©������Һ��
��2������ȩ��������������CH3COOH���²�Һ�����ԣ���������ʯ����Һ�����²�Һ�Ƿ�����ԣ��������Ϊ��ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�������
�ʴ�Ϊ��ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�������
��3��������������ʹ�ӷ����ļӺ���Һ�����������뵽��ƿ�ڣ������ȱ�֤����ȩ�Ĵ��ȣ�Ҳ�����ԭ�ϵ������ʣ�����ʱ��ȴˮ�ķ����������������෴��Ӧ���ǵ�ʱ�߳���
�ʴ�Ϊ��ʹ�Ӻ���������������ƿ�ڣ�b��
�����ݴ��µ�������Һ������У����Խ��ԽС��ֱ����ȫ��ʧ��˵����ȩ������ˮ�����۲쵽��������������Сʱ��Ҫ�����ܴ���Һ�� ȡ�����Է�ֹ������
�ʴ�Ϊ��������ˮ����ʱ��ȥ���ܣ���ֹ������
���� ���⿼������ȩ�Ļ�ѧ���ʣ��ۺ��Խ�ǿ���漰�����ķ�����ᴿ��ʵ����������ȩ�����ʵȣ��Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �����K��N���ӣ������������ḯʴ | |
| B�� | �����K��N���ӣ�������Ӧʽ��4OH--4e-�T2H2O+O2�� | |
| C�� | �����K��M���ӣ���ʯī������ͭ������ʵ�������϶�ͭ | |
| D�� | �����K��M���ӣ�������������22.4L����״��������ʱ��������1mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����һ��������С��Ԫ����Cu����Ԫ�ط��ţ���
����һ��������С��Ԫ����Cu����Ԫ�ط��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ƾ���ʹ�����ʱ��ԣ�ҽѧ��ʹ����ˮ�ƾ���ɱ������ | |
| B�� | NH3����ˮ���Լ��ԣ���FeCl3������Һ��ͨ������NH3����ȡFe��OH��3���� | |
| C�� | ClO2����ǿ�����ԣ�����������ˮ��ɱ�������������������ĸ�Ч��ȫ�� | |
| D�� | Na2CO3�������ᷴӦ���㷺��������θ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
�����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����� | ���������� | �ܵ��� | ����� |
| ���ڸ�������� | �ۢ� | �ڢߢ�� | �ݢޢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com