
��6�֣�ij��������Ʒ�к����������Ͷ����������ʣ�������ȡ��������������ijͬѧ������µ�ʵ�鷽�����ش��������⣺
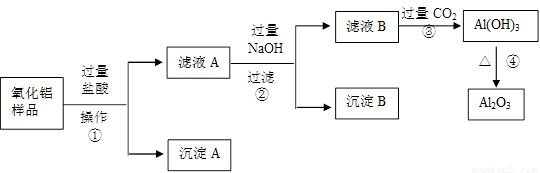
��1�������ٵ����� ��
��2������A�Ļ�ѧʽ��___________����ҺB�е������ӳ���Cl����OH�����______________��
��3������ܵĻ�ѧ����ʽΪ______________________________________��
��4���ڲ�������Ҫ�õ��IJ������������ձ���������������_________________��
���������������費�������ᣬͨ�����˼��õ�����A����Һ�к��е��ǹ��������ᣬ���ɵ��Ȼ������Ȼ������������������������Һ���Ȼ����������������������Ȼ�������ƫ�����ƣ����˼��õ�����������������ʱ��Һ�к��й��˵��������ƺ�����ƫ�������Լ��Ȼ��ƣ�ͨ������Ķ�����̼�����������������������������������ȶ������ȷֽ�������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
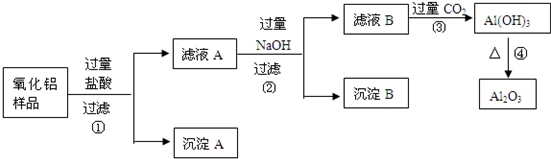
ij��������Ʒ�к����������Ͷ����������ʣ����������費�������ᣩ������ȡ��������������ijͬѧ������µ�ʵ�鷽�����ش��������⣺
��1������A�Ļ�ѧʽ��________________������B�Ļ�ѧʽ��________________����ҺA�к��е�������Ϊ �� ����ҺB�еĺ���Ԫ�صĻ����ﻯѧʽ��_________ ____��
��2��������У��ӹ������������Ʒ�����������Ӧ�û�ѧ����ʽ��ʾΪ��
��
��
��3������۵����ӷ���ʽΪ ��
�����ӵ�����Ϊ���������ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ��
��
��4���ڲ�������Ҫ�õ��IJ��������� ��___________��__________��
��5������ܵķ�Ӧ��ѧ����ʽΪ_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�긣������ܼ����ѧ�߶���ѧ�����п����Ŀƻ�ѧ�Ծ����������� ���ͣ�ʵ����
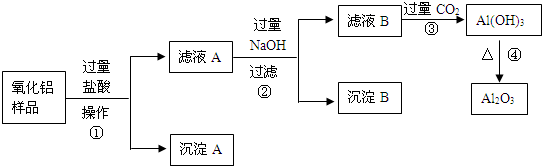
��6�֣�ij��������Ʒ�к����������Ͷ����������ʣ�������ȡ��������������ijͬѧ������µ�ʵ�鷽�����ش��������⣺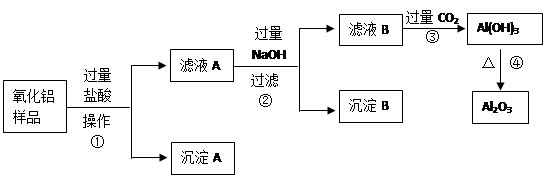
��1�������ٵ����� ��
��2������A�Ļ�ѧʽ��___________����ҺB�е������ӳ���Cl����OH�����______________��
��3������ܵĻ�ѧ����ʽΪ______________________________________��
��4���ڲ�������Ҫ�õ��IJ������������ձ���������������_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2009-2010ѧ�����ѧ�ڸ�һ3���¿���ѧ���� ���ͣ�������
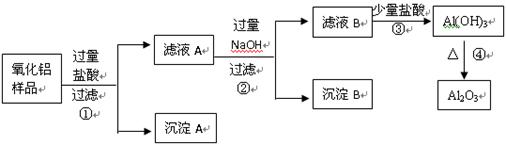
��18�֣�ij��������Ʒ�к����������Ͷ����������ʣ����������費�������ᣩ������ȡ��������������ijͬѧ������µ�ʵ�鷽�����ش��������⣺

��1������A�Ļ�ѧʽ��________________������B�Ļ�ѧʽ��________________����ҺA�к��е�������Ϊ �� ����ҺB�еĺ���Ԫ�صĻ����ﻯѧʽ��_________ ____��
��2��������У��ӹ������������Ʒ�����������Ӧ�û�ѧ����ʽ��ʾΪ��
��
��
��3������۵����ӷ���ʽΪ ��
�����ӵ�����Ϊ���������ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ��
��
��4���ڲ�������Ҫ�õ��IJ��������� ��___________��__________��
��5������ܵķ�Ӧ��ѧ����ʽΪ_____________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com