
±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ����dz��ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽�� Һ�ֲ㣬��Ƽ�㷽�����жϺ�Ϊ��ˮ�㡱 ��
Һ�ֲ㣬��Ƽ�㷽�����жϺ�Ϊ��ˮ�㡱 ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ ��
��5����A�����NaOH�Ҵ���Һ�������ɵ�����ͨ��������Ȼ�̼��Һ�У���Һ��ɫ��ԭ���� ��
��1��Br ��1�֣�
��2����ȡһ��Һ�壬��ˮ����������ã������ֲ㣬�ò�Ϊ��ˮ�㡱����֮Ϊ���Ͳ㡱����1�֣�
��3��������NaOH��Һ������������Һ��Ӧ���ɳ������Ӷ�����X-�ļ��顣 ��2�֣�
��4��ϡ���ᡣ ��1�֣�
CH3CH2Br+NaOH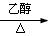 CH2=CH2��+NaBr+H2O ��3�֣�
CH2=CH2��+NaBr+H2O ��3�֣�
HNO3+NaOH=NaNO3+H2O ��3�֣�
NaBr+AgNO3=AgBr��+NaNO3 ��3�֣�
��5��A�����NaOH �Ҵ���Һ�������ɵ�����������ϩ ��1�֣�
�Ҵ���Һ�������ɵ�����������ϩ ��1�֣�
����


 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ����dz��ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽��Һ�ֲ㣬��Ƽ�㷽�����жϺ�Ϊ��ˮ�㡱 ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ ��
��5����A�����NaOH�Ҵ���Һ�������ɵ�����ͨ��������Ȼ�̼��Һ�У���Һ��ɫ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������«����һ����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ���ѡ��
±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ���ð�ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽�������� ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������и���8���¿������Ծ����������� ���ͣ�ʵ����
��16�֣�±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ����dz��ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽��Һ�ֲ㣬��Ƽ�㷽�����жϺ�Ϊ��ˮ�㡱 ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ ��
��5����A�����NaOH�Ҵ���Һ�������ɵ�����ͨ��������Ȼ�̼��Һ�У���Һ��ɫ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��������«����һ����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
±����A��C2H5X����һ����ɫҺ�壬Ϊ̽��A�����ʣ��������ʵ�鷽����
����һ����A�м�����������Һ����������á�
����������A�мӹ���NaOHˮ��Һ����������ã���Һ��ֲ��ȡ��ˮ�㡱��Һ�������μ���������Һ��
����������A�м������NaOH�Ҵ���Һ�����ȣ���ַ�Ӧ��ȡ��Һ���������μ����Լ�B����������Һ���ð�ɫ������
����������Ϣ�ش����⡣
��1��C2H5X�е�X�� �� ��д��ѧʽ��
��2������һ�пɹ۲쵽�������� ��
��3��������Ϊ�������ﲻ������X-��ʵ��Ŀ�ģ������� ��
��4���������У��Լ�B�� ��д���������п��ܷ�����Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com