��֪��A��ʯ���ѽ�������Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־���������л���A��G֮���ת����ϵ��
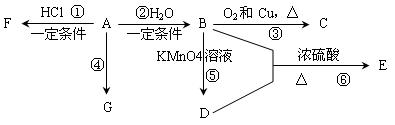
��ش��������⣺
��1��A�Ĺ����ŵ�������________________��C�Ľṹ��ʽ��________________��
��2��E��һ�־�����ζ��Һ�壬��B + D���ķ�Ӧ����ʽΪ��___________________________________���÷�Ӧ������_______________��
��γ�ȥE��������������D���ʣ������������̣�________________________________________
______________________________________________________________________________________��
��3��G��һ�ָ߷��ӻ������������_____________��������______________��
��4�������У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ��������F���е�12.27OC������Ӧ��������д����A��F�Ļ�ѧ��Ӧ����ʽ��________________________________���÷�Ӧ��ԭ��������Ϊ__________������F�������䶳����Ӧ��������������________________________________
__________________________________________��


 ��У����ϵ�д�
��У����ϵ�д�
 ��֪��A��ʯ���ѽ�������Ҫ�ɷݣ���ʹ��ˮ��ɫ��A��һ����Ҫ�Ļ���ԭ�ϣ����IJ���ͨ����������һ������ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ������������߷��ӻ�����E����ϳ�·����ͼ��ʾ����ش��������⣺
��֪��A��ʯ���ѽ�������Ҫ�ɷݣ���ʹ��ˮ��ɫ��A��һ����Ҫ�Ļ���ԭ�ϣ����IJ���ͨ����������һ������ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ������������߷��ӻ�����E����ϳ�·����ͼ��ʾ����ش��������⣺






 ��
��
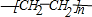
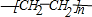
 +2Na��
+2Na�� +H2��
+H2�� +2Na��
+2Na�� +H2��
+H2��






