


| 9.0��10-12 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O
Cr2O72-+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
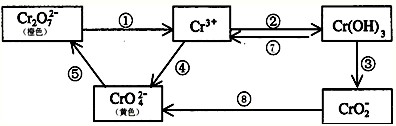
 ��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����
��2010?��ͷһģ�������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ���� Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O
Cr2O72-+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 Cr2O72-+H2O
Cr2O72-+H2O Cr2O72-+H2O
Cr2O72-+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭��������������֯�߶���ѧ������������ѧ�Ծ��������棩 ���ͣ������
��10�֣������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����

�ش��������⣺
��1��������Ӧ���������������� (����)��
��2����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,��̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ:
�������������������������������������� ��������������������������������������
��Һ��������������������������������������
��3����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10����������23�и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
��7�֣��������������Ԫ�أ��纬�������Ӱ�������֬��Ĵ�л����������������ж����йغ�����������ת����ϵ����

�ش��������⣺
��1����ԭ�ӵĵ����Ų�ʽ����������������������������������������������
��2��������Ӧ���������������� �����ţ���
��3����ҵ�ϴ�����Cr2O72-�ķ�ˮʱ��һ�㽫�綾��Cr2O72-ת��ΪCr3+,д����̼Ϊ����������������������NaCl��Cr2O72-�����Է�ˮ��д���缫��Ӧ����Һ�н��еķ�Ӧ�����ӷ���ʽ����������������������������������������������������
����Һ����������������������������������������������
��4����Ӧ���ǿ��淴Ӧ����Na2CrO4��Һ�м���ϡ���ᣬ��Һ�ɻ�ɫ��ɳ�ɫ��д���÷�Ӧ�����ӷ���ʽ��������������������������������������������������
��5����֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ9.0��10��12��1.56��10��10��������ͬŨ�ȵ�Na2CrO4��NaCl�Ļ����Һ����μ�����������Һ���������ɵij�������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com