
��NAΪ���� �ӵ�������ֵ������˵����ȷ����
�ӵ�������ֵ������˵����ȷ����
A��һ��������������Fe����Ũ���ᷴӦ��ת�Ƶ�����һ��Ϊ3NA
B�����³�ѹ�£�15 g��(��CH3)������������Ϊ6NA
C����1 L��̼������Һ�У���c(CO )��1 mol��L��1����Na������ΪNA
)��1 mol��L��1����Na������ΪNA
D����4NA�����ӵĹ���Na2O2����ˮ���1 L��Һ��������Һ��Na ����Ũ��Ϊ1 mol��L��1
����Ũ��Ϊ1 mol��L��1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����о���ѧ�Ļ���������ͼ����ʾ��ʵ�鷽����װ�û��������ȷ����

A������SO2
B��ʵ������ȡ���ռ�O2
C��װ�������Եļ��
D�������Ҵ�������Ļ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ���л���ѧ����]3�Լױ���ϩ�����(E)��һ�����ںϳɿ�Ѫ˨ҩ���м��壬��ϳ�·�����£�

��֪��HCHO��CH3CHO CH2===CHCHO��H2O
CH2===CHCHO��H2O
(1)��FeCl3��Һ����ɫ�ұ�����������ȡ������A��ͬ���칹����____________�֣�B�к��������ŵ�����Ϊ________��
(2)�Լ�C��ѡ�������е�________��
a����ˮ b��������Һ
c������KMnO4��Һ d������Cu(OH)2����Һ
 |
(3) ��E��һ��ͬ���칹�壬������������NaOH��Һ���ȵĻ�ѧ����ʽΪ____________________��
(4)E��һ�������¿������ɸ߾���F��F�Ľṹ��ʽΪ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ӱ������еõ�һ����A(C10H16)���Ц�����������A��ط�Ӧ���£�

 | |||
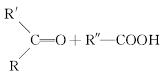 | |||
��֪�� 
(1)H�ķ���ʽΪ____________��
(2)B���������ŵ�����Ϊ____________��
(3)��������COOCH3���ŵ�C��ͬ���칹�干��________��(�����������칹)�����к˴Ź������׳���2�����շ���칹��Ľṹ��ʽΪ______________��
(4)B��D��D��E�ķ�Ӧ���ͷֱ�Ϊ______________��____________��
(5)GΪ����Ԫ���Ļ����д����ṹ��ʽ��______________��
(6)F��һ�������·����ۺϷ�Ӧ�ɵõ�һ�ָ���ˮ����֬������֬����Ϊ___________��
(7)д��E��F�Ļ�ѧ��Ӧ����ʽ��_________________________��
(8)A�Ľṹ��ʽΪ____________��A������ʵ�����Br2���мӳɷ�Ӧ�IJ��ﹲ��________��(�����������칹)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������˵����ȷ����
A��NA��Fe(OH)3�������ӵ�����Ϊ107 g
B��8.0 g Cu2S�� CuO�Ļ�����к���ͭԭ����Ϊ0.1NA
CuO�Ļ�����к���ͭԭ����Ϊ0.1NA
C����״���£���2.24 L Cl2����ˮ���ɵõ�HClO���ӵ���Ŀ��0.1NA
D��2.3 g Na��������ȫ��Ӧ����Ӧ��ת�Ƶĵ���������0.1NA��0.2NA֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����
A��6.4 g CaC2�к������ӵ���ĿΪ0.3NA
B�����³�ѹ�£�0.1 mol��L��1��Na2CO3��Һ�к���CO С��0.1NA
��0.1NA
C����״���£�11.2 L��ϩ�к��ЦҼ�Ϊ4NA
D��56 g����ϡ���ᷴӦ��ȫ��ת�Ƶ�����һ��Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ǵ��������ı����������������и�ǿ�������ԡ�ʵ���ҿɽ�����ͨ����ѹ�ŵ������ȡ������3O2 2O3��
2O3��
(1)����������Ӧ����30%������ת��Ϊ���������û������ƽ��Ħ������Ϊ________ g��mol��1(����һλС��)��
(2) ��8 L����ͨ���ŵ�ָܺ���ԭ״�����õ�����6.5 L�����г���Ϊ________ L��
��8 L����ͨ���ŵ�ָܺ���ԭ״�����õ�����6.5 L�����г���Ϊ________ L��
(3)ʵ���ҽ������ͳ����Ļ������0.896 L(��״��)ͨ��ʢ��20.0 gͭ�۵ķ�Ӧ���У���ּ��Ⱥ�ĩ��������Ϊ21.6 g����ԭ������г������������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ϊ10 L�Ĺ̶��ܱ�������ͨ��3 mol X���壬��һ���¶��·������·�Ӧ��
2X(g)  Y(g)��3Z(g)��
Y(g)��3Z(g)��
(1)��5 min��Ӧ�ﵽƽ�⣬��ʱ��������ڵ�ѹǿΪ��ʼʱ��1.2��������Y��ʾ�ķ�Ӧ����Ϊ________mol/(L��min)��
(2)��������Ӧ�ڼס��ҡ��������ĸ�ͬ�����ܱ������н��У���ͬһ��ʱ���ڲ�������ڵķ�Ӧ���ʷֱ�Ϊ��
| ���� | ��Ӧ���� |
| �� | v(X)��3.5 mol/(L��min) |
| �� | v(Y)��2 mol/(L��min) |
| �� | v(Z)��4.5 mol/(L��min) |
| �� | v(X)��0.075 mol/(L��s) |
������������ͬ���¶Ȳ�ͬ�����¶��ɸߵ��͵�˳����(�����)________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com