
(1998ğȫ¹ś£¬35)ĻĀĆęŹĒĖÄÖÖŃĪŌŚ²»Ķ¬ĪĀ¶ČĻĀµÄČܽā¶Č(g/100 g H2O)
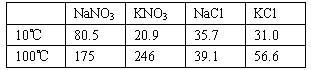
(¼ĘĖ揱¼Ł¶Ø£ŗ¢ŁŃĪĄą¹²“ꏱ²»Ó°Ļģø÷×ŌµÄČܽā¶Č£»¢Ś¹żĀĖ¾§ĢåŹ±£¬ČܼĮĖšŗÄŗöĀŌ²»¼Ę”£)
(1)Č”23.4 g NaClŗĶ40.4 g KNO3£¬¼Ó70.0 g H2O£¬¼ÓČČČܽā”£ŌŚ100”ꏱÕō·¢µō50.0 g H2O£¬Ī¬³ÖøĆĪĀ¶Č£¬¹żĀĖĪö³ö¾§Ģ壬¼ĘĖćĖłµĆ¾§ĢåµÄÖŹĮæ(![]() )”£
)ӣ
½«ĀĖŅŗĄäČ“ÖĮ10”ę£¬“ż³ä·Ö½į¾§ŗ󣬹żĀĖ”£¼ĘĖćĖłµĆ¾§ĢåµÄÖŹĮæ(![]() )”£
)ӣ
(2)ĮķČ”34.0 g NaNO3ŗĶ29.8 g KCl£¬Ķ¬Ńł½ųŠŠČēÉĻŹµŃ锣10”ꏱĪö³öµÄ¾§ĢåŹĒ (Š“»ÆѧŹ½)”£100”ęŗĶ10”ęµĆµ½µÄ¾§ĢåÖŹĮæ(m”äøßĪĀŗĶm”äµĶĪĀ)·Ö±šŹĒ¶ąÉŁ?
 ¼ā×ÓÉśŠĀæĪĢĆæĪŹ±×÷ŅµĻµĮŠ“š°ø
¼ā×ÓÉśŠĀæĪĢĆæĪŹ±×÷ŅµĻµĮŠ“š°ø Ó¢²Å¼Ę»®Ķ¬²½æĪŹ±øߊ§ŃµĮ·ĻµĮŠ“š°ø
Ó¢²Å¼Ę»®Ķ¬²½æĪŹ±øߊ§ŃµĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ038
(2004”¤Č«¹ś)æĘѧ¼Ņ·¢ĻÖijŅ©ĪļMÄÜÖĪĮĘŠÄŃŖ¹Ü¼²²”£¬ŹĒŅņĪŖĖüŌŚČĖĢåÄŚÄÜŹĶ·Å³öŅ»ÖÖ”°ŠÅŹ¹·Ö×ÓD”±£¬²¢²ūĆ÷ĮĖDŌŚČĖĢåÄŚµÄ×÷ÓĆŌĄķ£®ĪŖ“ĖĖūĆĒČŁ»ńĮĖ1998ÄźÅµ±“¶ūÉśĄķѧŗĶŅ½Ń§½±£®
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŅŃÖŖMµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ227£¬ÓÉC”¢H”¢O”¢NĖÄÖÖŌŖĖŲ×é³É£¬C”¢H”¢NµÄÖŹĮæ·ÖŹżŅĄ“ĪĪŖ15.86£„”¢2.20£„ŗĶ18.50£„£¬ŌņMµÄ·Ö×ÓŹ½ŹĒ_______________________£®DŹĒĖ«Ō×Ó·Ö×Ó£¬·Ö×ÓĮæĪŖ30£¬ŌņDµÄ·Ö×ÓŹ½ĪŖ____________________________________________________£®
(2)ÓĶÖ¬A¾ĻĀĮŠĶ¾¾¶æɵƵ½M£®
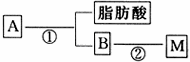
Ķ¼ÖŠ¢ŚµÄĢįŹ¾£ŗ

·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ________________________________£®
·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ__________________________________£®
(3)CŹĒBŗĶŅŅĖįŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³ÉµÄ»ÆŗĻĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ134£¬Š“³öCĖłÓŠæÉÄܵĽį¹¹¼ņŹ½_____________________________________£®
(4)Čō½«0.1molBÓė×ćĮæµÄ½šŹōÄĘ·“Ó¦£¬ŌņŠčĻūŗÄ____________________g½šŹōÄĘ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø04ğȫ¹ś¾ķ£©£Øl4·Ö£© æĘѧ¼Ņ·¢ĻÖijŅ©ĪļMÄÜÖĪĮĘŠÄŃŖ¹Ü¼²²”ŹĒŅņĪŖĖüŌŚČĖĢåÄŚÄÜŹĶ·Å³öŅ»ÖÖ”°ŠÅŹ¹·Ö×Ó”±D£¬²¢²ūĆ÷ĮĖDŌŚČĖĢåÄŚµÄ×÷ÓĆŌĄķ”£ĪŖ“ĖĖūĆĒČŁ»ńĮĖ1998ÄźÅµ±“¶ūÉśĄķѧ»ņŅ½Ń§½±”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖMµÄ·Ö×ÓĮæĪŖ227£¬ÓÉC”¢H”¢O”¢NÖÜĖÄÖÖŌŖĖŲ×é³É£¬C”¢H”¢NµÄÖŹĮæ·ÖŹżŅĄ“ĪĪŖ15.86%”¢2.20%ŗĶ18.50%”£ŌņMµÄ·Ö×ÓŹ½ŹĒ_____________________”£DŹĒĖ«Ō×Ó·Ö×Ó£¬·Ö×ÓĮæĪŖ30£¬ŌņDµÄ·Ö×ÓŹ½ĪŖ________________”£
£Ø2£©ÓĶÖ¬A¾ĻĀĮŠĶ¾¾¶æɵƵ½M”£

·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ_________________________________________________________________________”£
·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ_________________________________________________________________________”£
£Ø3£©CŹĒBŗĶŅŅĖįŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³ÉµÄ»ÆŗĻĪļ£¬·Ö×ÓĮæĪŖ134£¬Š“³öCĖłÓŠæÉÄܵĽį¹¹¼ņŹ½
____________________________________ӣ
£Ø4£©Čō½«0.1mol BÓė×ćĮæµÄ½šŹōÄĘ·“Ó¦£¬ŌņŠčĻūŗÄ_________g½šŹōÄĘ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A.ŠæĮ£ÓėĻ”HNO3·“Ó¦ÖʱøH2
B.Ļņ±„ŗĶNaClČÜŅŗÖŠµĪ¼ÓÅØH2SO4ÖʱøHCl
C.ŃĒĮņĖįÄĘÓėÅØH2SO4·“Ó¦ÖʱøSO2
D.“óĄķŹÆÓėÅØH2SO4·“Ó¦ÖʱøCO2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A.1”Ć4 B.1”Ć5 C.2”Ć1 D.2”Ć3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A.“ĪĀČĖįøĘČÜŅŗÖŠĶØČė¹żĮ涞Ńõ»ÆĢ¼£ŗCa2+£«2ClO££«H2O£«CO2====CaCO3”ż£«2HClO
B.ĮņĖįŃĒĢśČÜŅŗÖŠ¼Ó¹żŃõ»ÆĒāČÜŅŗ£ŗFe2+£«2H2O2£«4H+====Fe3+£«4H2O
C.ÓĆ°±Ė®ĪüŹÕÉŁĮ涞Ńõ»ÆĮņ£ŗNH3”¤H2O£«SO2====![]() £«
£«![]()
D.ĻõĖįĢśČÜŅŗÖŠ¼Ó¹żĮæ°±Ė®£ŗFe3£«£«3NH3”¤H2O ====Fe£ØOH£©3”ż£«3![]()
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com